Organic Chemistry

UNDERSTANDING CARBON-BASED SUBSTANCES AND DEVELOPING ECONOMIC, GREEN STRATEGIES TO PRODUCE USEFUL NEW MOLECULES, REACTIONS AND MATERIALS
Stanford chemists are developing more efficient and sustainable chemistries by exploring the structure, properties and reactions of organic compounds and materials. New reagents and catalysts are enabling greener industrial processes. Growing understanding of natural product properties, activities and synthesis are leading to potential new therapeutics, in close collaborations with researchers in the School of Medicine. These cutting-edge efforts build on a strong departmental history in organic synthesis.

Organic Synthesis
Invention of new tools and methods make it possible to create complex molecules from simple starting materials, more rapidly and cost-efficiently. Stanford chemists are developing new methods to synthesize target molecules with potential applications as novel catalysts, antibiotics and antitumor therapies ; atomically efficient methods to create new transition-metal-based non-protein catalysts ; new atom and group transfer-type reaction processes for natural product synthesis and chemical biology; and novel approaches to the design and synthesis of exotic small and giant molecules for custom properties . Elusive, selective reactions at the boundaries of modern organic synthesis take inspiration from natural products – and answer questions about their properties and activities.
Molecular Design
Stanford chemists are crafted designers of a wide variety of molecules for applications in chemical synthesis, materials science, and biomedicine. Advancements in synthetic capabilities and efficiency allow for freedom of molecular design. Stanford chemists are designing new reactions, catalysts, and reagents for more efficient, selective, and robust chemical transformations; new molecular strategies to develop more effective drugs; new imaging agents, optical reporters, and molecular delivery vehicles to allow integration of biological systems and delivery of therapeutics into cells; new classes of biological probes for the study of cell surface glycans; fluorescent probes of DNA repair enzymes in cells and tissues; and novel classes of unusual molecular and polymeric materials with tailored optical, electronic, thermal, and mechanical properties.
Green Chemistries
Using mechanistic principles to develop new catalytic strategies, Stanford chemists synthesize complex, useful macromolecular architectures, including sustainable polymers, synthetic fuels, and bioactive molecules ; and develop cost-efficient catalysts and chemical reactions that recycle CO2 into fuels and commodity chemicals using renewable energy sources. To understand and reproduce the remarkable specificity and energy efficiency of metalloenzymes, Stanford chemist are studying the mechanism of dioxygen activation by copper-containing enzymes.
Biomedicine
Stanford researchers are employing organic methods to explore the roles of cell-surface sugars and glycosylation in health, aging and illness , including cancer; to study and engineer enzymatic assembly lines that catalyze the biosynthesis of antibiotics in bacteria; and to design nucleotides with unusual properties such as fluorescence, enzyme reactivity, or altered shape and bonding ability, as tools to study nucleotide function and potential new probes for cancer diagnosis.
Computer Modeling
Computer studies of target molecules with desirable properties are finding ways to create functionally similar species that require fewer steps to synthesize – a technique called function oriented design and synthesis.
Associated Faculty

Steven Banik

Carolyn Bertozzi

James K. Chen

Justin Du Bois

Matthew Kanan

Chaitan Khosla

Daniel Stack

Robert Waymouth

Paul Wender

- Staff & Faculty Directory
- Disability Accommodations
- Diversity, Equity, and Inclusion Committee
- Major Awards
- Our Community Values
- Our History
- Quality of Life Committee
- Areas of Research
- Facilities and Centers
- Instructors
- Postdoctoral Research and Resources
- Graduate Program
- Undergraduate Programs
- Chemistry Undergraduate Teaching Laboratory
- Our Chemistry Education Office
- Elementary Schools
- High Schools
- Community Relations and Outreach
- Contact our Development Officer
- Funds to Support
- Meet Our Major Supporters
Organic research includes the invention of new reactions as well as investigations at the interface of organic chemistry with biology, medicine, materials science, and nanotechnology. Specific areas of interest include the development of new catalytic processes and methods for continuous flow synthesis, the design and synthesis of macromolecules, and the total synthesis of bioactive natural products.

Related News

Department of Chemistry hosts the Third Annual Future Faculty Symposium
The event showcased the research of early-career scientists who are committed to diversity, equity, and inclusion in STEM.
MIT chemists synthesize plant-derived molecules that hold potential as pharmaceuticals
Large multi-ring-containing molecules known as oligocyclotryptamines have never been produced in the lab until now.

Robert Gilliard wins 2024 Cottrell SEED Award
This competitive prize supports members of the Cottrell Scholar community in high-impact research activities.

Scientists preserve DNA in an amber-like polymer
With their “T-REX” method, DNA embedded in the polymer could be used for long-term storage of genomes or digital data such as photos and music.

Jeremiah Johnson and Heather Kulik among the winners of the Royal Society of Chemistry’s 2024 Materials Chemistry Horizon Prize
The prize was given for demonstrating the potential and impact of embedded mechanochemical reactivity on the mechanical limits of cross-linked polymer networks.

QS ranks MIT the world’s No. 1 university for 2024-25
Ranking at the top for the 13th year in a row, the Institute also places first in 11 subject areas.

Xiao Wang earns Tenure
Wang has been promoted to Associate Professor with Tenure, effective July 1, 2024.

Congratulations to the Class of 2024
26 undergraduate Chemistry Majors were awarded their degrees on Friday, May 31, 2024.
- Utility Menu
gA4 tracking code
Department of chemistry and chemical biology.
- Donate to CCB
Organic Chemistry
CCB's long and illustrious history of accomplishment in the area of carbon-containing compounds is exemplified by pioneering syntheses of the complex molecules palytoxin and vitamin B12.
With emphasis on development of new reaction methodology and synthesis of families of molecules ranging from small probes to large compounds with antimicrobial and antibiotic activity, and creation of supramolecular assemblies of relevance to energy conversion, Harvard’s organic chemistry effort continues to be multifaceted and fruitful.
Organic Chemistry Faculty

Emily Balskus

E. J. Corey

Eric Jacobsen

David R. Liu

Richard Y. Liu

Andrew Myers

Daniel G. Nocera

Stuart L. Schreiber

Matthew Shair

Christina Woo
Recent news.

Brian Liau receives 2025 Eli Lilly Award

Christina Woo Granted Tenure

Eric Jacobsen receives the 2024 Welch Award in Chemistry
Browse by research area.
Department of Chemistry
- Organic Chemistry
- Chemical Biology
- Inorganic Chemistry
- Materials Chemistry
- Physical Chemistry
- Theoretical Chemistry
Organic chemistry at JHU bridges both synthetic and physical organic chemistry. Current activities include research in synthetic methodology, materials, natural products, medicinal chemistry, and chemical biology. Our recent research has found application in medicine (Lectka, Toscano), biology (Greenberg, Rokita, Townsend), and materials science (Klausen, Tovar).
Groups: Greenberg , Huang , Klausen , Lectka , Rokita , Toscano , Tovar , Townsend
Group Highlights
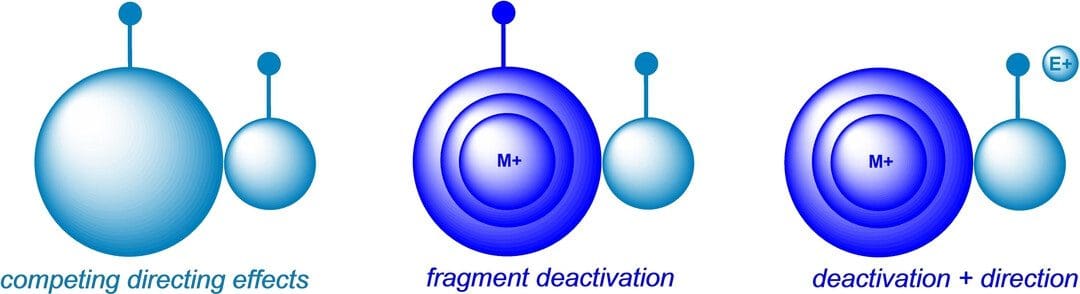
Metal Ion-Induced Large Fragment Deactivation
Complex natural product functionalizations generally involve the use of highly engineered reagents, catalysts, or enzymes to achieve site-selectivity by lowering a selected transition state energy. We invert this strategy by […]
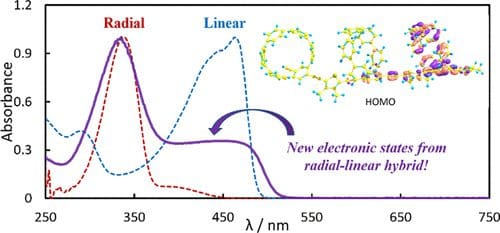
Linear and Radial Conjugation in Extended π-Electron Systems
We describe the synthesis and electronic properties of new π-conjugated small molecules and polymers that combine the linear intramolecular conjugation pathways commonly associated with organic electronic materials with the emerging […]
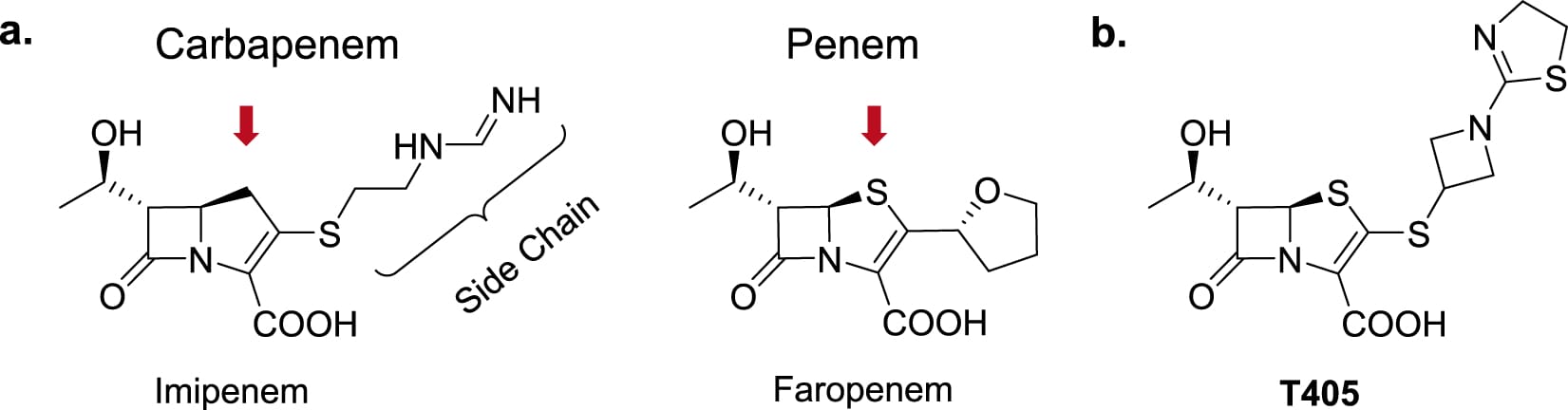
Development of a penem antibiotic against Mycobacteroides abscessus
Pulmonary disease caused by Mycobacteriodes abscessus has outpaced tuberculosis in the U.S., with cure rates below 50 percent. This sparked collaboration between the Townsend Research Lab and two School of […]
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
- hot-topics Extras
- Newsletters
- Reading room
Tell us what you think. Take part in our reader survey
Celebrating twenty years
- Back to parent navigation item
- Collections
- Chemistry of the brain
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- Energy storage and batteries
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus
- More navigation items
Organic chemistry
The latest chemistry news and research on organic chemistry, including synthesis, natural products and total synthesis, reaction mechanisms and supramolecular chemistry, from the Royal Society of Chemistry's magazine, Chemistry World
Being wrong is almost inevitable
2024-08-28T15:38:00Z
On the tightrope of expressing your opinion, you always risk looking a fool

China cracks down on fentanyl precursor chemicals
2024-08-14T08:30:00Z
Three fentanyl chemical ingredients will be subject to new restrictions from September
Medicinal chemistry’s biological blind spots
2024-08-08T11:15:00Z
Despite advances in modern medicine, there are some big gaps in our knowledge

Chemical ‘waves’ used to encode words as Morse code
2024-08-06T13:33:00Z
A controllable, oscillating reaction has been used to generate waves of different forms for information transduction

The power of a printed chart
2024-07-25T13:30:00Z
Even in this online era, some things are still best kept on paper
Designer ylide transfers single carbon atoms
2024-07-24T13:30:00Z
Single-atom carbon transfer reagent provides the latest breakthrough in skeletal editing
Forgotten borole synthesis expands family of antiaromatic compounds
2024-07-23T11:26:00Z
Study investigates how benzene-fused borole compares to its non-fused and doubly-fused cousins

Electrochemical reaction outcomes controlled by customised AC waveforms
2024-07-17T10:12:00Z
Programmed alternating currents allow researchers to choose between reaction mechanisms with the flick of a switch

Super-fast automated synthesis promises to make chemistry accessible to many more
2024-07-04T08:30:00Z
Order of magnitude improvement in speed result of technology optimisation
Harnessing carbene reactivity with light
2024-07-03T08:50:00Z
Two-step photocatalytic strategy produces metal carbenes easily and safely

Stability milestone for nitrene
2024-06-27T09:30:00Z
Bench-stable transition metal–nitrene complexes beckon
Photochemistry sheds light on the direct functionalisation of native sugars
2024-06-27T08:30:00Z
New radical reaction reduces the number of protection and deprotection steps needed, accelerating discovery of sugar compounds

Pillar[5]arene gets fully furnished with bulky aromatics
2024-06-21T09:47:00Z
Synthesis developed by pillararene pioneers overcomes steric hindrance while preserving macrocycle’s pillar-shaped structure

From pollutant to painkiller: hazardous halogenated wastes become a safe chlorine source
2024-06-20T08:30:00Z
Persistent pollutants can be used to produce painkillers
Intermediate considered ‘too reactive to isolate’ finally tamed
2024-06-19T13:30:00Z
Nitrene species stabilised for three days with careful choice of substituent


Sparking industry’s interest in electrosynthesis
2024-06-17T09:23:00Z
Using electrons instead of reagents offers many potential benefits, but there are still barriers to overcome, as Rachel Brazil reports
(–)-Bipolarolide D
2024-06-12T08:30:00Z
A rare example of a [6 + 2]-cycloaddition

Clever activation strategy widens access to phosphazene superbases
2024-06-11T08:57:00Z
Superbases stored as stable carboxylate salts – adding epoxide releases their full chemical potential
Study questions sustainability benefits of replacing palladium with nickel in cross-coupling reactions
2024-06-06T08:38:00Z
Common belief that Earth-abundant metals are green replacements for palladium may be misleading

Will science ever reach an end?
2024-05-30T11:00:00Z
While the rate of discoveries in any field may slow over time, the frontier creeps ever further
- Previous Page
- Contributors
- Terms of use
- Accessibility
- Permissions
- This website collects cookies to deliver a better user experience. See how this site uses cookies .
- This website collects cookies to deliver a better user experience. Do not sell my personal data .
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies .
Site powered by Webvision Cloud
- Department Resources
- Department Calendar
DEPARTMENT OF CHEMISTRY
- Areas of Research
Organic Chemistry
Organic chemistry research at Northwestern University covers many scientific areas, both those traditionally associated with the field and in areas that impact other disciplines. These research areas range from the synthesis of bioactive small molecules to the formation of functional extended polymers or nanostructures, and from understanding how light waves interact with organic matter to exploring the chemistry of proteins, cells or living organisms. As such, faculty and students within the organic chemistry division have diverse research interests that intersect with many of the most pressing issues and needs facing modern society. Whether it be developing new medicines to treat disease or inventing useful materials for solar energy conversion or environmental remediation, the organic chemistry division is dedicated to conducting forward-looking research and training the next generation of scientific leaders.
Organic Chemistry Research Areas:
Chemical biology .
Molecular mechanisms of action, design and syntheses of bioactive small-molecules • neurodegenerative diseases • proteomics • bioorganic chemistry • natural product bio- synthesis and discovery • cell adhesion
Farha , Gianneschi , Kelleher , Mrksich , Nguyen , Scheidt , Silverman , Stupp , Thomson
Organic Nanotechnology & Materials
Supramolecular chemistry • mechanostereochemistry • molecular recognition • self-assembly • functionalized and mechanized molecules • metal-organic frameworks and porous organic materials • artificial photosynthesis
Dichtel , Kalow , Malapit , Mrksich , Nguyen , Stoddart , Stupp , Wasielewski
Physical Organic Chemistry
Electron donor–acceptor systems • photochemistry • molecular electronics • surface chemistry of graphene and polymer nanocomposites • self-assembled monolayers • mass spectrometry
Dichtel , Kalow , Malapit , Nguyen , Wasielewski
Total Synthesis & Method Development
Natural product synthesis • alkaloids • polyketides • polycyclic molecules • organocatalysis • asymmetric catalysis • organometallic chemistry • polymers • new reaction discovery and development
Dichtel , Kalow , Malapit , Marks , Nguyen , Scheidt , Thomson

Current Organic Chemistry
Special Promotion To Our Authors There will be no Publication Charges on submitting articles till 31st December, 2024
Impact Factor : 1.7
Indexed in: Scopus, SCI Expanded, JCR... View all
Volume 28 , Issues 20, 2024
This journal supports open access
Submission for General Articles
Submit to thematic issues.

Thematic Issue Issue: {[{issue.issue_title}]}
{[{issue.about_issue}]}
No Text Found
- Submit Abstracts
- Submit Manuscripts Online
- Thematic Issue Proposal
- Animated Abstract Submission

- About Journal
- Editorial Board
- Journal Insight
- Current Issue
- Volumes /Issues
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
- Announcements
- Forthcoming Thematic Issues
- Guest Editor Guidelines
- Editorial Management
- Ethical Guidelines for New Editors
- Reviewer Guidelines
- Abstract Ahead of Print 0
- Article(s) in Press 24
- Free Online Copy
- Most Cited Articles
- Most Accessed Articles
- Highlighted Article
- Most Popular Articles
- Editor's Choice
- Thematic Issues
- Open Access Articles
- Open Access Funding
- Library Recommendation
- Trial Requests
- Advertise With Us
- Meet the Executive Guest Editor(s)
- Brand Ambassador
- Author's Comment & Reviews
- New Journals 2023
- New Journals 2024
- Alert Subscription

Organic Synthesis
Research in the Ritter group focuses on the development of novel reaction chemistry. We seek to discover molecular structure and reactivity that can contribute to interdisciplinary solutions for challenges in science. The lab focuses on synthetic organic and organometallic chemistry, complex molecule synthesis, and mechanistic studies to develop practical access to molecules of interest in catalysis, medicine, and materials.
Research Topics:
Late-stage functionalization, late-stage fluorination, unappreciated redox reactivity of palladium.

How does a research institute actually work?
Students from a local school get interesting insights at the MPI more

Safer alternative for an explosive reaction
The chemical industry has been using a reaction with explosive chemicals for over 100 years - now Mülheim scientists have discovered a safer alternative. more

Thiel Award 2024 goes to Philipp Hartmann
Philipp Hartmann, PhD candidate in the group of Prof. Tobias Ritter, has been awarded the Thiel Award. Each year the Max-Planck-Institut für Kohlenforschung honors a young talent in the field of chemistry with this prestigious prize. more

Thianthrenium chemistry allows for reactivity switch of a nucleophilic amino acid into a versatile intermediate
Ritter group publishes their findings with “Nature Chemistry” more

Teenagers experience extraordinary holidays
Local school students visit the institute for a whole week more
Working in the radionuclide laboratory
Research report:.
- Research Report Ritter 2017-2019 523.46 kB
- Research Report Ritter 2014-2016 1.16 MB

- Resources for Support
- Prospective Ph.D. Students
- How to Apply
- Grad Program FAQ
- Current Graduate Students
- Graduate Student Organizations
- Program Contacts
- About Our Program
- Undergrad Program FAQ
- Academic Planning & Advising
- Teaching, Mentoring & Outreach
- Undergraduate Research
- Awards & Scholarships
- Graduate Students
- Postdoctoral Scholars
- Career Network and Recruitment Fair
- Postdoc-to-PI Symposium 2024
- Analytical Research
- Biological Research
- Energy and Environmental Research
- Inorganic Research
- Materials Research
Organic Research
- Physical Research
- Theoretical Research
- Eberly Fellows
- Research Facilities
- NMR Facility
- Institutes and Centers
- Mission Statement
- Climate & Diversity Committee
- Chemistry Department Guides
- Wellness Space
- Recognition Months
- DEI Resources
- Fellowships
Dept. of Department of Chemistry
The Penn State Department of Chemistry has a growing legacy in organic and small molecule methodology and complex synthesis. Faculty are engaged in cutting edge research that spans the realm of traditional synthesis and methodology, polymer chemistry, electrochemical methods, stimuli-responsive reactivity, and bioorganic chemistry. Researchers have interests that are centered in new methodology for the synthesis of molecules and materials for utility in pharmaceuticals, catalysis, active/responsive networks, biomedicine, and electronic materials.

Harry Allcock Evan Pugh University Professor of Chemistry Polymer synthesis, materials chemistry, and biomedicine
Bert Chandler Professor of Chemistry and of Chemical Engineering Environmental Catalysis; Nanoparticle & Materials Synthesis; Catalytic Reaction Mechanisms
Elizabeth Elacqua Assistant Professor of Chemistry Small molecule and polymer synthesis, supramolecular catalysis, and organic molecules under confinement
Julie Fenton Assistant Professor of Chemistry Materials synthesis and characterization; inorganic, organic, and hybrid solids; colloidal nanomaterials; surface chemistry.
Ray Funk Professor of Chemistry Development of new synthetic methodology with emphasis on ring construction
Ramesh Giri Weinreb Early Career Professor of Chemistry New synthetic methods, catalysis, organometallic chemistry, and sustainable chemistry
Jonathan Kuo Assistant Professor of Chemistry Physical Organic, Organometallic, Bioinorganic
Eric Nacsa Assistant Professor of Chemistry New activation strategies and synthetic methods
Ayusman Sen Verne M. Willaman Professor of Chemistry Homogeneous catalysis, polymeric materials
Steven Weinreb Professor Emeritus Total synthesis of natural products
Ruobo Zhou Assistant Professor of Chemistry Super-resolution fluorescence imaging, single-molecule detection, spatiotemporal organizations of biomolecules, liquid-liquid phase separation in biology, neurobiology, RNA biology.
Adjunct Faculty
Xin Zhang Design and synthesis of small molecules to image and modulate intracellular protein aggregates
Department of Chemistry
College of Natural & Agricultural Sciences

Organic Chemistry
Research in Organic Chemistry at UC Riverside spans the whole range of topics in modern organic chemistry, from biological and medicinal chemistry to natural product synthesis, the discovery of new reactions and materials to the physical organic chemistry of reaction mechanisms. In addition, our faculty have interests in supramolecular self-assembly, the creation of functional materials and study of reactivity at the solid interface. We have active collaborations with other disciplines in chemistry (such as Analytical, Biological, Inorganic and Physical) as well as other departments at UC Riverside such as Chemical Genomics, Materials Science and Engineering, Biochemistry, Plant Biology and Entomology. Please follow the links below to learn more about the individual research groups in the organic chemistry program at UC Riverside.
Subjects of first-year organic graduate courses include modern organic reactions and reagents and their mechanistic pathways, structure and bonding in organic compounds, kinetics and mechanism of organic reactions, synthetic organic chemistry and spectroscopic identification of organic compounds. Weekly seminars in both the department and the research group familiarize students with current research topics.
Faculty Research Descriptions:
Ana bahamonde.
We focus on the design and investigation of asymmetric catalytic reactions. We use physical organic chemistry tools, organometallic complexes and visible light to enable challenging bond disconnections.
Matthew Casselman
My scholarly activity focuses on implementing and assessing the effectiveness of evidence-based instructional practices, primarily in large-enrollment organic chemistry courses.
Hill Harman
Synthetic Inorganic, Organometallic and Organic chemistry: ligand and catalyst design; organocatalysis; electrocatalysis; structure, bonding, and reactivity of the transition and main group metals; energy and green chemistry.
Richard Hooley
Synthetic Organic, Inorganic and Supramolecular chemistry. Our projects include: the synthesis of biomimetic supramolecular constructs capable of selective molecular recognition; synthesis of new water-soluble catalysts and host molecules; dynamic NMR studies of host guest interactions; biosensors based on synthetic receptor molecules.
Synthetic Organic Chemistry, Organometallic Chemistry, catalysis and its applications to synthesis, natural product synthesis, and mechanistic investigations.
Catharine Larsen
Organic and Organometallic chemistry; discovery of new multicomponent metal-mediated reactions and their applications to the synthesis of complex molecules.
Vince Lavallo
Synthetic Organometallic, Inorganic and Organic chemistry. The preparation of novel transition metal and non-metallic catalysts for a variety of industrially important chemical transformations. Ligand design, asymmetric catalysis, new reaction methodology and carborane chemistry.
Jocelyn Millar
Identification and synthesis of insect pheromones and related behavior modifying chemicals; identification of Kairomones; development of applications of pheromones and related compounds for agricultural crop protection.
William Neary
The Neary group at the University of California, Riverside specializes in the metathesis polymerization of nonconventional substrates. We aim to overcome current state-of-the-art polymerization limitations through rational monomer design and systematic catalyst-monomer pairing. The successful generation of these microstructures, once thought to be inaccessible, will lead to materials with applications in sensing, recycling, and lithium-ion battery formulations.
Michael Pirrung
Chemical biology, synthetic organic chemistry, nucleic acids, combinatorial chemistry; photochemistry.
Organic-focused projects in the Su Lab involve the chemical synthesis of organic electronic materials such as π-conjugated molecules and polymers. In some projects, chemical synthesis is followed by measurement in the lab's homebuilt STM-BJ instrument, where quantum transport characteristics in molecular wires are dictated by fundamental concepts from physical organic chemistry.
Christopher Switzer
Design, synthesis and characterization of nucleic acid variants with new properties for molecular recognition, catalysis and replication.
Kathryn Uhrich
Coming Soon!
The Xue group exploits the art of organic chemistry to develop protein mimetic multicyclic peptides. These peptides grant access to a true 3D-diversifiable chemical space, which promises novel chemical biology tools and better therapeutics.
Organic & Biomolecular Chemistry
Introduction to computational organic chemistry.

a Yusuf Hamied Department of Chemistry, Lensfield Road, Cambridge CB2 1EW, UK
b Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada
c Department of Chemistry, University of Houston, Houston, TX 77204, USA
A graphical abstract is available for this content

- This article is part of the themed collection: Computational Organic Chemistry
Article information
Download citation, permissions.
J. M. Goodman, J. P. Reid and J. I. Wu, Org. Biomol. Chem. , 2024, Advance Article , DOI: 10.1039/D4OB90102A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 21 August 2019
Synthetic organic chemistry driven by artificial intelligence
- A. Filipa de Almeida ORCID: orcid.org/0000-0002-8399-0710 1 ,
- Rui Moreira 1 &
- Tiago Rodrigues ORCID: orcid.org/0000-0002-1581-5654 2
Nature Reviews Chemistry volume 3 , pages 589–604 ( 2019 ) Cite this article
13k Accesses
185 Citations
50 Altmetric
Metrics details
- Cheminformatics
- Computational chemistry
- Organic chemistry
- Chemical synthesis
Synthetic organic chemistry underpins several areas of chemistry, including drug discovery, chemical biology, materials science and engineering. However, the execution of complex chemical syntheses in itself requires expert knowledge, usually acquired over many years of study and hands-on laboratory practice. The development of technologies with potential to streamline and automate chemical synthesis is a half-century-old endeavour yet to be fulfilled. Renewed interest in artificial intelligence (AI), driven by improved computing power, data availability and algorithms, is overturning the limited success previously obtained. In this Review, we discuss the recent impact of AI on different tasks of synthetic chemistry and dissect selected examples from the literature. By examining the underlying concepts, we aim to demystify AI for bench chemists in order that they may embrace it as a tool rather than fear it as a competitor, spur future research by pinpointing the gaps in knowledge and delineate how chemical AI will run in the era of digital chemistry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
SAVI, in silico generation of billions of easily synthesizable compounds through expert-system type rules
Automation and computer-assisted planning for chemical synthesis
A large-scale reaction dataset of mechanistic pathways of organic reactions
Nantermet, P. G. Reaction: the art of synthetic chemistry. Chem 1 , 335–336 (2016).
CAS Google Scholar
Nicolaou, K. C. & Chen, J. S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 38 , 2993–3009 (2009).
CAS PubMed PubMed Central Google Scholar
Baran, P. S. Natural product total synthesis: as exciting as ever and here to stay. J. Am. Chem. Soc. 140 , 4751–4755 (2018).
CAS PubMed Google Scholar
Ley, S. V. The engineering of chemical synthesis: humans and machines working in harmony. Angew. Chem. Int. Ed. 57 , 5182–5183 (2018).
Bergman, R. G. & Danheiser, R. L. Reproducibility in chemical research. Angew. Chem. Int. Ed. 55 , 12548–12549 (2016).
Duros, V. et al. Human versus robots in the discovery and crystallization of gigantic polyoxometalates. Angew. Chem. Int. Ed. 56 , 10815–10820 (2017).
Roch, L. M. et al. ChemOS: Orchestrating autonomous experimentation. Science Robot. 3 , eaat5559 (2018).
Google Scholar
Schneider, G. Mind and machine in drug design. Nat. Mach. Intell. 1 , 128–130 (2019).
Wang, Y. et al. Acoustic droplet ejection enabled automated reaction scouting. ACS Cent. Sci. 5 , 451–457 (2019).
Fitzpatrick, D. E., Battilocchio, C. & Ley, S. V. Enabling technologies for the future of chemical synthesis. ACS Cent. Sci. 2 , 131–138 (2016).
Ley, S. V., Fitzpatrick, D. E., Myers, R. M., Battilocchio, C. & Ingham, R. J. Machine-assisted organic synthesis. Angew. Chem. Int. Ed. 54 , 10122–10136 (2015).
Lehmann, J. W., Blair, D. J. & Burke, M. D. Toward generalization of iterative small molecule synthesis. Nat. Rev. Chem. 2 , 0115 (2018).
PubMed PubMed Central Google Scholar
Corey, E. J. & Wipke, W. T. Computer-assisted design of complex organic syntheses. Science 166 , 178–192 (1969).
Pensak, D. A. & Corey, E. J. in Computer-Assisted Organic Synthesis Ch. 1 (eds Wipke, W. T. & Howe, W. J.) 1-32 (American Chemical Society, 1977).
Lajiness, M. S., Maggiora, G. M. & Shanmugasundaram, V. Assessment of the consistency of medicinal chemists in reviewing sets of compounds. J. Med. Chem. 47 , 4891–4896 (2004).
Earkin, D. R. & Warr, W. A. in Computer-Assisted Organic Synthesis Ch. 10 (eds Wipke, W. T. & Howe, W. J.) 217-226 (American Chemical Society, 1977).
Sridharan, N. S. in Computer-Assisted Organic Synthesis Ch. 7 (eds Wipke, W. T. & Howe, W. J.) 148-178 (American Chemical Society, 1977).
Wipke, W. T., Ouchi, G. I. & Krishnan, S. Simulation and evaluation of chemical synthesis—SECS: An application of artificial intelligence techniques. Artif. Intell. 11 , 173–193 (1978).
Hessler, G. & Baringhaus, K. H. Artificial intelligence in drug design. Molecules 23 , E2520 (2018).
PubMed Google Scholar
Sellwood, M. A., Ahmed, M., Segler, M. H. & Brown, N. Artificial intelligence in drug discovery. Future Med. Chem. 10 , 2025–2028 (2018).
Aspuru-Guzik, A., Lindh, R. & Reiher, M. The matter simulation (r)evolution. ACS Cent. Sci. 4 , 144–152 (2018).
Lusher, S. J., McGuire, R., van Schaik, R. C., Nicholson, C. D. & de Vlieg, J. Data-driven medicinal chemistry in the era of big data. Drug Discov. Today 19 , 859–868 (2014).
Tetko, I. V., Engkvist, O., Koch, U., Reymond, J. L. & Chen, H. BIGCHEM: challenges and opportunities for big data analysis in chemistry. Mol. Inf. 35 , 615–621 (2016).
Henson, A. B., Gromski, P. S. & Cronin, L. Designing algorithms to aid discovery by chemical robots. ACS Cent. Sci. 4 , 793–804 (2018).
Rich, A. S. & Gureckis, T. M. Lessons for artificial intelligence from the study of natural stupidity. Nat. Mach. Intell. 1 , 174–180 (2019).
Ekins, S. et al. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 18 , 435–441 (2019).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46 , D1074–D1082 (2018).
Gaulton, A. et al. The ChEMBL database in 2017. Nucleic Acids Res. 45 , D945–D954 (2017).
Kim, S. et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 47 , D1102–D1109 (2019).
Grzybowski, B. A. et al. Chematica: A story of computer code that started to think like a chemist. Chem 4 , 390–398 (2018).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555 , 604–610 (2018).
Schneider, N., Lowe, D. M., Sayle, R. A., Tarselli, M. A. & Landrum, G. A. Big data from pharmaceutical patents: A computational analysis of medicinal chemists’ bread and butter. J. Med. Chem. 59 , 4385–4402 (2016).
Ahneman, D. T., Estrada, J. G., Lin, S., Dreher, S. D. & Doyle, A. G. Predicting reaction performance in C–N cross-coupling using machine learning. Science 360 , 186–190 (2018).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54 , 3451–3479 (2011).
Lowe, D. AI designs organic syntheses. Nature 555 , 592–593 (2018).
Coley, C. W., Green, W. H. & Jensen, K. F. Machine learning in computer-aided synthesis planning. Acc. Chem. Res. 51 , 1281–1289 (2018).
Gelernter, H. L. et al. Empirical explorations of SYNCHEM. Science 197 , 1041–1049 (1977).
Cadeddu, A., Wylie, E. K., Jurczak, J., Wampler-Doty, M. & Grzybowski, B. A. Organic chemistry as a language and the implications of chemical linguistics for structural and retrosynthetic analyses. Angew. Chem. Int. Ed. 53 , 8108–8112 (2014).
Coley, C. W., Rogers, L., Green, W. H. & Jensen, K. F. Computer-assisted retrosynthesis based on molecular similarity. ACS Cent. Sci. 3 , 1237–1245 (2017).
Hartenfeller, M. et al. DOGS: reaction-driven de novo design of bioactive compounds. PLoS Comput. Biol. 8 , e1002380 (2012).
Rodrigues, T. et al. De novo design and optimization of Aurora A kinase inhibitors. Chem. Sci. 4 , 1229–1233 (2013).
Rodrigues, T. et al. Steering target selectivity and potency by fragment-based de novo drug design. Angew. Chem. Int. Ed. 52 , 10006–10009 (2013).
Friedrich, L., Rodrigues, T., Neuhaus, C. S., Schneider, P. & Schneider, G. From complex natural products to simple synthetic mimetics by computational de novo design. Angew. Chem. Int. Ed. 55 , 6789–6792 (2016).
Lewell, X. Q., Judd, D. B., Watson, S. P. & Hann, M. M. RECAP — retrosynthetic combinatorial analysis procedure: a powerful new technique for identifying privileged molecular fragments with useful applications in combinatorial chemistry. J. Chem. Inf. Comput. Sci. 38 , 511–522 (1998).
Reker, D., Bernardes, G. J. L. & Rodrigues, T. Computational advances in combating colloidal aggregation in drug discovery. Nat. Chem. 11 , 402–418 (2019).
Liu, B. et al. Retrosynthetic reaction prediction using neural sequence-to-sequence models. ACS Cent. Sci. 3 , 1103–1113 (2017).
Altae-Tran, H., Ramsundar, B., Pappu, A. S. & Pande, V. Low data drug discovery with one-shot learning. ACS Cent. Sci. 3 , 283–293 (2017).
Chen, H., Engkvist, O., Wang, Y., Olivecrona, M. & Blaschke, T. The rise of deep learning in drug discovery. Drug Discov. Today 23 , 1241–1250 (2018).
Ching, T. et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 15 , 20170387 (2018).
Baylon, J. L., Cilfone, N. A., Gulcher, J. R. & Chittenden, T. W. Enhancing retrosynthetic reaction prediction with deep learning using multiscale reaction classification. J. Chem. Inf. Model. 59 , 673–688 (2019).
Fialkowski, M., Bishop, K. J., Chubukov, V. A., Campbell, C. J. & Grzybowski, B. A. Architecture and evolution of organic chemistry. Angew. Chem. Int. Ed. 44 , 7263–7269 (2005).
Gothard, C. M. et al. Rewiring chemistry: algorithmic discovery and experimental validation of one-pot reactions in the network of organic chemistry. Angew. Chem. Int. Ed. 51 , 7922–7927 (2012).
Grzybowski, B. A., Bishop, K. J., Kowalczyk, B. & Wilmer, C. E. The ‘wired’ universe of organic chemistry. Nat. Chem. 1 , 31–36 (2009).
Kowalik, M. et al. Parallel optimization of synthetic pathways within the network of organic chemistry. Angew. Chem. Int. Ed. 51 , 7928–7932 (2012).
Segler, M. H. S. & Waller, M. P. Neural-symbolic machine learning for retrosynthesis and reaction prediction. Chem. Eur. J. 23 , 5966–5971 (2017).
Silver, D. et al. Mastering the game of Go with deep neural networks and tree search. Nature 529 , 484–489 (2016).
Browne, C. et al. A survey of Monte Carlo tree search methods. IEEE Trans. Comput. Intell. AI Games 4 , 1–43 (2012).
Schreck, J. S., Coley, C. W. & Bishop, K. J. M. Learning retrosynthetic planning through simulated experience. ACS Cent. Sci. 5 , 970–981 (2019).
Szymkuc, S. et al. Computer-assisted synthetic planning: The end of the beginning. Angew. Chem. Int. Ed. 55 , 5904–5937 (2016).
Klucznik, T. et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4 , 522–532 (2018).
Molga, K., Dittwald, P. & Grzybowski, B. A. Navigating around patented routes by preserving specific motifs along computer-planned retrosynthetic pathways. Chem 5 , 460–473 (2019).
Badowski, T., Molga, K. & Grzybowski, B. A. Selection of cost-effective yet chemically diverse pathways from the networks of computer-generated retrosynthetic plans. Chem. Sci. 10 , 4640–4651 (2019).
Burke, K. Perspective on density functional theory. J. Chem. Phys. 136 , 150901 (2012).
Chermette, H. Chemical reactivity indexes in density functional theory. J. Comput. Chem. 20 , 129–154 (1999).
Hegde, G. & Bowen, R. C. Machine-learned approximations to density functional theory Hamiltonians. Sci. Rep. 7 , 42669 (2017).
Smith, J. S., Isayev, O. & Roitberg, A. E. ANI-1: an extensible neural network potential with DFT accuracy at force field computational cost. Chem. Sci. 8 , 3192–3203 (2017).
Grisafi, A. et al. Transferable machine-learning model of the electron density. ACS Cent. Sci. 5 , 57–64 (2019).
Sadowski, P., Fooshee, D., Subrahmanya, N. & Baldi, P. Synergies between quantum mechanics and machine learning in reaction prediction. J. Chem. Inf. Model. 56 , 2125–2128 (2016).
Moosavi, S. M. et al. Capturing chemical intuition in synthesis of metal-organic frameworks. Nat. Commun. 10 , 539 (2019).
Raccuglia, P. et al. Machine-learning-assisted materials discovery using failed experiments. Nature 533 , 73–76 (2016).
Kayala, M. A., Azencott, C. A., Chen, J. H. & Baldi, P. Learning to predict chemical reactions. J. Chem. Inf. Model. 51 , 2209–2222 (2011).
Fooshee, D. et al. Deep learning for chemical reaction prediction. Mol. Syst. Des. Eng. 3 , 442–452 (2018).
Schwaller, P., Gaudin, T., Lanyi, D., Bekas, C. & Laino, T. “Found in Translation”: predicting outcomes of complex organic chemistry reactions using neural sequence-to-sequence models. Chem. Sci. 9 , 6091–6098 (2018).
Wei, J. N., Duvenaud, D. & Aspuru-Guzik, A. Neural networks for the prediction of organic chemistry reactions. ACS Cent. Sci. 2 , 725–732 (2016).
Hughes, T. B., Dang, N. L., Miller, G. P. & Swamidass, S. J. Modeling reactivity to biological macromolecules with a deep multitask network. ACS Cent. Sci. 2 , 529–537 (2016).
Hughes, T. B., Miller, G. P. & Swamidass, S. J. Modeling epoxidation of drug-like molecules with a deep machine learning network. ACS Cent. Sci. 1 , 168–180 (2015).
Coley, C. W., Barzilay, R., Jaakkola, T. S., Green, W. H. & Jensen, K. F. Prediction of organic reaction outcomes using machine learning. ACS Cent. Sci. 3 , 434–443 (2017).
Coley, C. W. et al. A graph-convolutional neural network model for the prediction of chemical reactivity. Chem. Sci. 10 , 370–377 (2019).
Breiman, L. Random forests. Mach. Learn. 45 , 5–32 (2001).
Ho, T. K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 20 , 832–844 (1998).
Rodrigues, T. et al. De novo fragment design for drug discovery and chemical biology. Angew. Chem. Int. Ed. 54 , 15079–15083 (2015).
Rodrigues, T. et al. Machine intelligence decrypts beta-lapachone as an allosteric 5-lipoxygenase inhibitor. Chem. Sci. 9 , 6899–6903 (2018).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545 , 299–304 (2017).
Wolfe, J. M. et al. Machine learning to predict cell-penetrating peptides for antisense delivery. ACS Cent. Sci. 4 , 512–520 (2018).
Chuang, K. V. & Keiser, M. J. Comment on “Predicting reaction performance in C–N cross-coupling using machine learning”. Science 362 , eaat8603 (2018).
Estrada, J. G., Ahneman, D. T., Sheridan, R. P., Dreher, S. D. & Doyle, A. G. Response to Comment on “Predicting reaction performance in C–N cross-coupling using machine learning”. Science 362 , eaat8763 (2018).
Skoraczynski, G. et al. Predicting the outcomes of organic reactions via machine learning: are current descriptors sufficient? Sci. Rep. 7 , 3582 (2017).
Chuang, K. V. & Keiser, M. J. Adversarial controls for scientific machine learning. ACS Chem. Biol. 13 , 2819–2821 (2018).
Beker, W., Gajewska, E. P., Badowski, T. & Grzybowski, B. A. Prediction of major regio-, site-, and diastereoisomers in diels-alder reactions by using machine-learning: the importance of physically meaningful descriptors. Angew. Chem. Int. Ed. 58 , 4515–4519 (2019).
Nielsen, M. K., Ahneman, D. T., Riera, O. & Doyle, A. G. Deoxyfluorination with sulfonyl fluorides: navigating reaction space with machine learning. J. Am. Chem. Soc. 140 , 5004–5008 (2018).
Halford, G. S., Baker, R., McCredden, J. E. & Bain, J. D. How many variables can humans process? Psychol. Sci. 16 , 70–76 (2005).
Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 652 , 161–172 (2009).
Murray, P. M. et al. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org. Biomol. Chem. 14 , 2373–2384 (2016).
Austin, N. D., Sahinidis, N. V., Konstantinov, I. A. & Trahan, D. W. COSMO-based computer-aided molecular/mixture design: A focus on reaction solvents. AIChE J. 63 , 104–122 (2018).
Struebing, H. et al. Computer-aided molecular design of solvents for accelerated reaction kinetics. Nat. Chem. 5 , 952–957 (2013).
Truhlar, D. G. Chemical reactivity: Inverse solvent design. Nat. Chem. 5 , 902–903 (2013).
Gao, H. et al. Using machine learning to predict suitable conditions for organic reactions. ACS Cent. Sci. 4 , 1465–1476 (2018).
Zhou, Z., Li, X. & Zare, R. N. Optimizing chemical reactions with deep reinforcement learning. ACS Cent. Sci. 3 , 1337–1344 (2017).
Bedard, A. C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361 , 1220–1225 (2018).
Reker, D. & Schneider, G. Active-learning strategies in computer-assisted drug discovery. Drug. Discov. Today 20 , 458–465 (2015).
Reker, D., Schneider, P. & Schneider, G. Multi-objective active machine learning rapidly improves structure–activity models and reveals new protein–protein interaction inhibitors. Chem. Sci. 7 , 3919–3927 (2016).
Reker, D. & Brown, J. B. Selection of informative examples in chemogenomic datasets. Methods Mol. Biol. 1825 , 369–410 (2018).
Reker, D., Schneider, P., Schneider, G. & Brown, J. B. Active learning for computational chemogenomics. Future Med. Chem. 9 , 381–402 (2017).
Sans, V., Porwol, L., Dragone, V. & Cronin, L. A self optimizing synthetic organic reactor system using real-time in-line NMR spectroscopy. Chem. Sci. 6 , 1258–1264 (2015).
Häse, F., Roch, L. M., Kreisbeck, C. & Aspuru-Guzik, A. Phoenics: A Bayesian optimizer for chemistry. ACS Cent. Sci. 4 , 1134–1145 (2018).
Frazier, P. I. A tutorial on Bayesian optimization. Preprint at arXiv https://arxiv.org/abs/1807.02811 (2018).
Brochu, E., Cora, V. M. & Freitas, N. d. A tutorial on Bayesian optimization of expensive cost functions, with application to active user modeling and hierarchical reinforcement learning. Preprint at arXiv https://arxiv.org/abs/1012.2599 (2010).
Reker, D., Bernardes, G. J. L. & Rodrigues, T. Evolving and nano data enabled machine intelligence for chemical reaction optimization. Preprint at ChemRxiv https://chemrxiv.org/articles/Evolving_and_Nano_Data_Enabled_Machine_Intelligence_for_Chemical_Reaction_Optimization/7291205/1 (2018).
Granda, J. M., Donina, L., Dragone, V., Long, D. L. & Cronin, L. Controlling an organic synthesis robot with machine learning to search for new reactivity. Nature 559 , 377–381 (2018).
Ahmadi, M., Vogt, M., Iyer, P., Bajorath, J. & Frohlich, H. Predicting potent compounds via model-based global optimization. J. Chem. Inf. Model. 53 , 553–559 (2013).
Patil, P. C. & Luzzio, F. A. Synthesis of extended oxazoles II: Reaction manifold of 2-(halomethyl)-4,5-diaryloxazoles. Tetrahedron Lett. 57 , 757–759 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10 , 383–394 (2018).
Roberts, R. M. Serendipity: Accidental Discoveries in Science 1-288 (John Wiley & Sons, 1989).
Davey, S. Rapid reaction discovery. Nat. Chem. 4 , 69 (2012).
McNally, A., Prier, C. K. & MacMillan, D. W. Discovery of an alpha-amino C–H arylation reaction using the strategy of accelerated serendipity. Science 334 , 1114–1117 (2011).
Amara, Z. et al. Automated serendipity with self-optimizing continuous-flow reactors. Eur. J. Org. Chem. 2015 , 6141–6145 (2015).
Dragone, V., Sans, V., Henson, A. B., Granda, J. M. & Cronin, L. An autonomous organic reaction search engine for chemical reactivity. Nat. Commun. 8 , 15733 (2017).
Gromski, P. S., Henson, A. B., Granda, J. M. & Cronin, L. How to explore chemical space using algorithms and automation. Nat. Rev. Chem. 3 , 119–128 (2019).
Cao, Y., Romero, J. & Aspuru-Guzik, A. Potential of quantum computing for drug discovery. IBM J. Res. Dev. 62 , 6:1–6:20 (2019).
Rodrigues, T. et al. Multidimensional de novo design reveals 5-HT2B2B receptor-selective ligands. Angew. Chem. Int. Ed. 54 , 1551–1555 (2015).
Reutlinger, M., Rodrigues, T., Schneider, P. & Schneider, G. Combining on-chip synthesis of a focused combinatorial library with computational target prediction reveals imidazopyridine GPCR ligands. Angew. Chem. Int. Ed. 53 , 582–585 (2014).
Ban, T. A. The role of serendipity in drug discovery. Dialogues Clin. Neurosci. 8 , 335–344 (2006).
Rosales, A. R. et al. Rapid virtual screening of enantioselective catalysts using CatVS. Nat. Catal. 2 , 41–45 (2019).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363 , eaav2211 (2019).
Caramelli, D. et al. Networking chemical robots for reaction multitasking. Nat. Commun. 9 , 3406 (2018).
Fitzpatrick, D. E., Maujean, T., Evans, A. C. & Ley, S. V. Across-the-world automated optimization and continuous-flow synthesis of pharmaceutical agents operating through a cloud-based server. Angew. Chem. Int. Ed. 57 , 15128–15132 (2018).
Lavecchia, A. Machine-learning approaches in drug discovery: methods and applications. Drug Discov. Today 20 , 318–331 (2015).
Butler, K. T., Davies, D. W., Cartwright, H., Isayev, O. & Walsh, A. Machine learning for molecular and materials science. Nature 559 , 547–555 (2018).
Jordan, M. I. & Mitchell, T. M. Machine learning: Trends, perspectives, and prospects. Science 349 , 255–260 (2015).
Sanchez-Lengeling, B. & Aspuru-Guzik, A. Inverse molecular design using machine learning: Generative models for matter engineering. Science 361 , 360–365 (2018).
Wallach, I. & Heifets, A. Most ligand-based classification benchmarks reward memorization rather than generalization. J. Chem. Inf. Model. 58 , 916–932 (2018).
Download references
Acknowledgements
A.F.A. acknowledges Fundação para a Ciência e Tecnologia (FCT) Portugal for financial support through a PhD grant (PD/BD/143125/2019). T.R. is an investigador auxiliar supported by FCT Portugal (CEECIND/00887/2017). T.R. acknowledges FCT/FEDER (02/SAICT/2017, grant 28333) for funding. The authors thank the reviewers for their comments.
Author information
Authors and affiliations.
Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
A. Filipa de Almeida & Rui Moreira
Instituto de Medicina Molecular (iMM) João Lobo Antunes, Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal
Tiago Rodrigues
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Tiago Rodrigues .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Chemistry thanks R. Lewis and B. Maryasin for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Area of computer science that deals with the recognition, processing and analysis of human (natural) language.
(SMILES arbitrary target specification). A notation for the accurate substructural feature identification and atom typing.
(Simplified molecular-input line-entry system). A notation to describe chemical structure using ASCII strings.
A method to map substructural information into a bit string. The bit length (size) and detail of encoded features are defined by the user.
A method to quantify similarity (ranging from 0 to 1) between molecules. Complete dissimilarity equates to 0 and full identity equals 1.
A method that normalizes a vector of length j into a probability distribution containing J probabilities in the interval [0,1]. The sum of all probabilities equals 1.0.
A machine-learning method giving a probability distribution over a number of possible functions. A prior belief regarding an event is refined through Bayesian inference as data builds up.
(LDA). A machine-learning method that finds linear combinations of features that separate classes, prior to dimensionality reduction and classification.
(SVM). A machine-learning method that separates data points in hyperspace through mathematical functions called kernels.
A method for fine-tuning a model trained on a larger set of related data. The method is employed when limited data are available to answer a research question.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
de Almeida, A.F., Moreira, R. & Rodrigues, T. Synthetic organic chemistry driven by artificial intelligence. Nat Rev Chem 3 , 589–604 (2019). https://doi.org/10.1038/s41570-019-0124-0
Download citation
Accepted : 19 July 2019
Published : 21 August 2019
Issue Date : October 2019
DOI : https://doi.org/10.1038/s41570-019-0124-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Computational drug development for membrane protein targets.
- Xiaolin Sun
- Horst Vogel
Nature Biotechnology (2024)
Artificial molecular pumps
- J. Fraser Stoddart
Nature Reviews Methods Primers (2024)
- Ramil Babazade
- Yousung Jung
Scientific Data (2024)
Molecular set representation learning
- Maria Boulougouri
- Pierre Vandergheynst
- Daniel Probst
Nature Machine Intelligence (2024)
Computational synthesis design for controlled degradation and revalorization
- Anna Żądło-Dobrowolska
- Karol Molga
- Bartosz A. Grzybowski
Nature Synthesis (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Organic chemistry articles from across Nature Portfolio Organic chemistry is the study of the synthesis, structure, reactivity and properties of the diverse group of chemical compounds primarily ...
Organic Chemistry in the News. Organic compounds, protein engineering, and more. Read all the latest research in the field of organic chemistry. Full-text with images. Free.
Read current and featured research from the The Journal of Organic Chemistry on ACS Publications, a trusted source for peer-reviewed journals.
Read the latest Research articles in Organic chemistry from Nature Chemistry
Organic Synthesis Invention of new tools and methods make it possible to create complex molecules from simple starting materials, more rapidly and cost-efficiently. Stanford chemists are developing new methods to synthesize target molecules with potential applications as novel catalysts, antibiotics and antitumor therapies; atomically efficient methods to create new transition-metal-based non ...
Check out the latest edition of the The Journal of Organic Chemistry on ACS Publications, a trusted source for peer-reviewed journals.
In organic chemistry, finding conditions that enable a broad range of compounds to undergo a particular type of reaction is highly desirable. However, conventional methods for doing so consume a ...
EurJOC (European Journal of Organic Chemistry) supports the global organic chemistry community by publishing high-quality international research covering all aspects of organic chemistry. Our research articles, reviews, concepts, and perspectives cover broad-scale topics such as new synthetic methods, total synthesis, mechanistic studies, and bioorganic, physical-organic, and supramolecular ...
Organic Organic research includes the invention of new reactions as well as investigations at the interface of organic chemistry with biology, medicine, materials science, and nanotechnology.
Read the latest articles of Current Organic Chemistry at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. [ 1] Study of structure determines their structural formula.
Electrochemistry in Synthetic Organic Chemistry. Read the latest articles of The Journal of Organic Chemistry at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Organic Chemistry. CCB's long and illustrious history of accomplishment in the area of carbon-containing compounds is exemplified by pioneering syntheses of the complex molecules palytoxin and vitamin B12. With emphasis on development of new reaction methodology and synthesis of families of molecules ranging from small probes to large compounds ...
Organic chemistry at JHU bridges both synthetic and physical organic chemistry. Current activities include research in synthetic methodology, materials, natural products, medicinal chemistry, and chemical biology.
The latest chemistry news and research on organic chemistry, including synthesis, natural products and total synthesis, reaction mechanisms and supramolecular chemistry, from ...
Organic Chemistry. Organic chemistry research at Northwestern University covers many scientific areas, both those traditionally associated with the field and in areas that impact other disciplines. These research areas range from the synthesis of bioactive small molecules to the formation of functional extended polymers or nanostructures, and ...
Read the latest Research articles in Organic chemistry from Scientific Reports
Part of a journal that explores the role of chemistry in our everyday lives, this section publishes significant fundamental and applied work across all branches of organic chemistry.
Current Organic Chemistry covers topics related to structures, properties and reactivities of synthetic and natural organic compounds, and reaction mechanisms.
Organic Synthesis. Research in the Ritter group focuses on the development of novel reaction chemistry. We seek to discover molecular structure and reactivity that can contribute to interdisciplinary solutions for challenges in science. The lab focuses on synthetic organic and organometallic chemistry, complex molecule synthesis, and ...
Organic Research. The Penn State Department of Chemistry has a growing legacy in organic and small molecule methodology and complex synthesis. Faculty are engaged in cutting edge research that spans the realm of traditional synthesis and methodology, polymer chemistry, electrochemical methods, stimuli-responsive reactivity, and bioorganic ...
Research in Organic Chemistry at UC Riverside spans the whole range of topics in modern organic chemistry, from biological and medicinal chemistry to natural product synthesis, the discovery of new reactions and materials to the physical organic chemistry of reaction mechanisms. In addition, our faculty have interests in supramolecular self ...
Jonathan Goodman, Jolene Reid and Judy Wu introduce the Organic & Biomolecular Chemistry themed collection on Computational Organic Chemistry. Computational Organic Chemistry
Chemistry - A European Journal showcases fundamental research and topical reviews in all areas of the chemical sciences around the world. Functional group interconversion is of great significance in organic synthesis.
Artificial intelligence has recently seen numerous applications in synthetic organic chemistry. Advanced pattern-recognition heuristics may facilitate the access to chemical matter of interest and ...
Asian Journal of Organic Chemistry. Accepted Articles e202400336. Research Article. FACILE AND METAL-FREE SYNTHESIS OF ISOFLAVONES USING α-ARYL-β,β-DITOSYLOXY KETONES ... Maharishi Markandeshwar (Deemed to be University) Institute of Medical Sciences and Research, Chemistry, INDIA. Search for more papers by this author. Rajesh Kumar, Rajesh ...
Angewandte Chemie International Edition is one of the prime chemistry journals in the world, publishing research articles, highlights, communications and reviews across all areas of chemistry. The electrochemical reduction of CO2 to high-value carbon-based chemicals provides a sustainable approach to achieving an artificial carbon cycle.
candidates to teach a range of courses in chemistry with a focus on organic chemistry and courses for non-majors. The department has a well-funded and maintained teaching laboratory with extensive instrumentation and facilities for a college of its size. Competitive internal funding opportunities are available for summer research grants, faculty
Read current and featured research from the The Journal of Organic Chemistry on ACS Publications, a trusted source for peer-reviewed journals. Recently Viewed close modal. Pair your accounts. ... Personal overviews of specialized research areas by acknowledged experts. Leveraging the Redox Promiscuity of Nickel To Catalyze C-N Coupling ...