- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
This article has a correction. Please see:
- New advances in type 1 diabetes - June 03, 2024
- Savitha Subramanian , professor of medicine ,
- Farah Khan , clinical associate professor of medicine ,
- Irl B Hirsch , professor of medicine
- University of Washington Diabetes Institute, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA, USA
- Correspondence to: I B Hirsch ihirsch{at}uw.edu
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to modify disease course and preserve β cell function have expanded our broad understanding of this condition. Biomarkers of type 1 diabetes are detectable months to years before development of overt disease, and three stages of diabetes are now recognized. The advent of continuous glucose monitoring and the newer automated insulin delivery systems have changed the landscape of type 1 diabetes management and are associated with improved glycated hemoglobin and decreased hypoglycemia. Adjunctive therapies such as sodium glucose cotransporter-1 inhibitors and glucagon-like peptide 1 receptor agonists may find use in management in the future. Despite these rapid advances in the field, people living in under-resourced parts of the world struggle to obtain necessities such as insulin, syringes, and blood glucose monitoring essential for managing this condition. This review covers recent developments in diagnosis and treatment and future directions in the broad field of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune condition that occurs as a result of destruction of the insulin producing β cells of the pancreatic islets, usually leading to severe endogenous insulin deficiency. 1 Without treatment, diabetic ketoacidosis will develop and eventually death will follow; thus, lifelong insulin therapy is needed for survival. Type 1 diabetes represents 5-10% of all diabetes, and diagnosis classically occurs in children but can also occur in adulthood. The burden of type 1 diabetes is expansive; it can result in long term complications, decreased life expectancy, and reduced quality of life and can add significant financial burden. Despite vast improvements in insulin, insulin delivery, and glucose monitoring technology, a large proportion of people with type 1 diabetes do not achieve glycemic goals. The massive burden of type 1 diabetes for patients and their families needs to be appreciated. The calculation and timing of prandial insulin dosing, often from food with unknown carbohydrate content, appropriate food and insulin dosing when exercising, and cost of therapy are all major challenges. The psychological realities of both acute management and the prospect of chronic complications add to the burden. Education programs and consistent surveillance for “diabetes burnout” are ideally available to everyone with type 1 diabetes.
In this review, we discuss recent developments in the rapidly changing landscape of type 1 diabetes and highlight aspects of current epidemiology and advances in diagnosis, technology, and management. We do not cover the breadth of complications of diabetes or certain unique scenarios including psychosocial aspects of type 1 diabetes management, management aspects specific to older adults, and β cell replacement therapies. Our review is intended for the clinical reader, including general internists, family practitioners, and endocrinologists, but we acknowledge the critical role that people living with type 1 diabetes and their families play in the ongoing efforts to understand this lifelong condition.
Sources and selection criteria
We did individual searches for studies on PubMed by using terms relevant to the specific topics covered in this review pertaining to type 1 diabetes. Search terms used included “type 1 diabetes” and each individual topic—diagnosis, autoantibodies, adjuvant therapies, continuous glucose monitoring, automated insulin delivery, immunotherapies, diabetic ketoacidosis, hypoglycemia, and under-resourced settings. We considered all studies published in the English language between 1 January 2001 and 31 January 2023. We selected publications outside of this timeline on the basis of relevance to each topic. We also supplemented our search strategy by a hand search of the references of key articles. We prioritized studies on each highlighted topic according to the level of evidence (randomized controlled trials (RCTs), systematic reviews and meta-analyses, consensus statements, and high quality observational studies), study size (we prioritized studies with at least 50 participants when available), and time of publication (we prioritized studies published since 2003 except for the landmark Diabetes Control and Complications Trial and a historical paper by Tuomi on diabetes autoantibodies, both from 1993). For topics on which evidence from RCTs was unavailable, we included other study types of the highest level of evidence available. To cover all important clinical aspects of the broad array of topics covered in this review, we included additional publications such as clinical reviews as appropriate on the basis of clinical relevance to both patients and clinicians in our opinion.
Epidemiology
The incidence of type 1 diabetes is rising worldwide, possibly owing to epigenetic and environmental factors. Globally in 2020 an estimated 8.7 million people were living with type 1 diabetes, of whom approximately 1.5 million were under 20 years of age. 2 This number is expected to rise to more than 17 million by 2040 ( https://www.t1dindex.org/#global ). The International Diabetes Federation estimates the global prevalence of type 1 diabetes at 0.1%, and this is likely an underestimation as diagnoses of type 1 diabetes in adults are often not accounted for. The incidence of adult onset type 1 diabetes is higher in Europe, especially in Nordic countries, and lowest in Asian countries. 3 Adult onset type 1 diabetes is also more prevalent in men than in women. An increase in prevalence in people under 20 years of age has been observed in several western cohorts including the US, 4 5 Netherlands, 6 Canada, 7 Hungary, 8 and Germany. 9
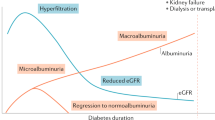
Classically, type 1 diabetes presents over the course of days or weeks in children and adolescents with polyuria, polydipsia, and weight loss due to glycosuria. The diagnosis is usually straightforward, with profound hyperglycemia (often >300 mg/dL) usually with ketonuria with or without ketoacidemia. Usually, more than one autoantibody is present at diagnosis ( table 1 ). 10 The number of islet autoantibodies combined with parameters of glucose tolerance now forms the basis of risk prediction for type 1 diabetes, with stage 3 being clinical disease ( fig 1 ). 11 The originally discovered autoantibody, islet cell antibody, is no longer used clinically owing to variability of the assay despite standardisation. 12
Autoantibody characteristics associated with increased risk of type 1 diabetes 10
- View inline

Natural history of type 1 diabetes. Adapted with permission from Insel RA, et al. Diabetes Care 2015;38:1964-74 11
- Download figure
- Open in new tab
- Download powerpoint
Half of all new cases of type 1 diabetes are now recognized as occurring in adults. 13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people. 14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable. The term latent autoimmune diabetes of adults (LADA) was introduced 30 years ago to identify adults who developed immune mediated diabetes. 15 An international consensus defined the diagnostic criteria for LADA as age >30 years, lack of need for insulin use for at least six months, and presence of islet cell autoantibodies. 16 However, debate as to whether the term LADA should even be used as a diagnostic term persists. The American Diabetes Association (ADA) Standards of Care note that for the purpose of classification, all forms of diabetes mediated by autoimmune β cell destruction are included in the classification of type 1 diabetes. 17 Nevertheless, they note that use of the term LADA is acceptable owing to the practical effect of heightening awareness of adults likely to have progressive autoimmune β cell destruction and thereby accelerating insulin initiation by clinicians to prevent diabetic ketoacidosis.
The investigation of adults with suspected type 1 diabetes is not always straightforward ( fig 2 ). 18 Islet cell autoantibodies such as glutamic acid decarboxylase antibody (GADA), tyrosine phosphatase IA2 antibody, and zinc transporter isoform 8 autoantibody act as markers of immune activity and can be detected in the blood with standardized assays ( table 1 ). The presence of one or more antibodies in adults with diabetes could mark the progression to severe insulin deficiency; these individuals should be considered to have type 1 diabetes. 1 Autoantibodies, especially GADA, should be measured only in people with clinically suspected type 1 diabetes, as low concentrations of GADA can be seen in type 2 diabetes and thus false positive measurements are a concern. 19 That 5-10% of cases of type 1 diabetes may occur without diabetes autoantibodies is also now clear, 20 and that the diabetes autoantibodies disappear over time is also well appreciated. 21

Flowchart for investigation of suspected type 1 diabetes in adults, based on data from white European populations. No single clinical feature in isolation confirms type 1 diabetes. The most discriminative feature is younger age at diagnosis (<35 years), with lower body mass index (<25), unintentional weight loss, ketoacidosis, and glucose >360 mg/dL at presentation. Adapted with permission from Holt RIG, et al. Diabetes Care 2021;44:2589-625 1
Genetic risk scoring (GRS) for type 1 diabetes has received attention to differentiate people whose classification is unclear. 22 23 24 Developed in 2019, the T1D-GRS2 uses 67 single nucleotide polymorphisms from known autoimmune loci and can predict type 1 diabetes in children of European and African ancestry. Although GRS is not available for routine clinical use, it may allow prediction of future cases of type 1 diabetes to allow prevention strategies with immune intervention (see below).
A major change in the type 1 diabetes phenotype has occurred over the past few decades, with an increase in obesity; the reasons for this are complex. In the general population, including people with type 1 diabetes, an epidemic of sedentary lifestyles and the “westernized diet” consisting of increased processed foods, refined sugars, and saturated fat is occurring. In people with type 1 diabetes, the overall improvement in glycemic control since the report of the Diabetes Control and Complications Trial (DCCT) in 1993 (when one or two insulin injections a day was standard therapy) has resulted in less glycosuria so that the typical patient with lower body weight is uncommon in high income countries. In the US T1D Exchange, more than two thirds of the adult population were overweight or obese. 25
Similarly, obesity in young people with type 1 diabetes has also increased over the decades. 26 The combination of autoimmune insulin deficiency with obesity and insulin resistance has received several descriptive names over the years, with this phenotype being described as double diabetes and hybrid diabetes, among others, 26 27 but no formal nomenclature in the diabetes classification exists. Many of these patients have family members with type 2 diabetes, and some patients probably do have both types of diabetes. Clinically, minimal research has been done into how this specific population responds to certain antihyperglycemic oral agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists, given the glycemic, weight loss, and cardiovascular benefits seen with these agents. 28 These patients are common in most adult diabetes practices, and weight management in the presence of insulin resistance and insulin deficiency remains unclear.
Advances in monitoring
The introduction of home blood glucose monitoring (BGM) more than 45 years ago was met with much skepticism until the report of the DCCT. 29 Since then, home BGM has improved in accuracy, precision, and ease of use. 30 Today, in many parts of the world, home BGM, a static measurement of blood glucose, has been replaced by continuous glucose monitoring (CGM), a dynamic view of glycemia. CGM is superior to home BGM for glycemic control, as confirmed in a meta-analysis of 21 studies and 2149 participants with type 1 diabetes in which CGM use significantly decreased glycated hemoglobin (HbA 1c ) concentrations compared with BGM (mean difference −0.23%, 95% confidence interval −3.83 to −1.08; P<0.001), with a greater benefit if baseline HbA 1c was >8% (mean difference −0.43%, −6.04 to −3.30; P<0.001). 31 This newer technology has also evolved into a critical component of automated insulin delivery. 32
CGM is the standard for glucose monitoring for most adults with type 1 diabetes. 1 This technology uses interstitial fluid glucose concentrations to estimate blood glucose. Two types of CGM are available. The first type, called “real time CGM”, provides a continuous stream of glucose data to a receiver, mobile application, smartwatch, or pump. The second type, “intermittently scanned CGM,” needs to be scanned by a reader device or smartphone. Both of these technologies have shown improvements in HbA 1c and amount of time spent in the hypoglycemic range compared with home BGM when used in conjunction with multiple daily injections or “open loop” insulin pump therapy. 33 34 Real time CGM has also been shown to reduce hypoglycemic burden in older adults with type 1 diabetes ( table 2 ). 36 Alerts that predict or alarm with both hypoglycemia and hyperglycemia can be customized for the patient’s situation (for example, a person with unawareness of hypoglycemia would have an alert at a higher glucose concentration). Family members can also remotely monitor glycemia and be alerted when appropriate. The accuracy of these devices has improved since their introduction in 2006, so that currently available sensors can be used without a confirmation glucose concentration to make a treatment decision with insulin. However, some situations require home BGM, especially when concerns exist that the CGM does not match symptoms of hypoglycemia.
Summary of trials for each topic covered
Analysis of CGM reports retrospectively can assist therapeutic decision making both for the provider and the patient. Importantly, assessing the retrospective reports and watching the CGM in real time together offer insight to the patient with regard to insulin dosing, food choices, and exercise. Patients should be encouraged to assess their data on a regular basis to better understand their diabetes self-management. Table 3 shows standard metrics and targets for CGM data. 52 Figure 3 shows an ambulatory glucose profile.
Standardized continuous glucose monitoring metrics for adults with diabetes 52

Example of ambulatory glucose profile of 52 year old woman with type 1 diabetes and fear of hypoglycemia. CGM=continuous glucose monitoring; GMI=glucose management indicator
Improvements in technology and evidence for CGM resulting in international recommendations for its widespread use have resulted in greater uptake by people with type 1 diabetes across the globe where available and accessible. Despite this, not everyone wishes to use it; some people find wearing any device too intrusive, and for many the cost is prohibitive. These people need at the very least before meal and bedtime home BGM.
A next generation implantable CGM device (Sensionics), with an improved calibration algorithm that lasts 180 days after insertion by a healthcare professional, is available in both the EU and US. Although fingerstick glucose calibration is needed, the accuracy is comparable to that of other available devices. 53
Advances in treatments
The discovery of insulin in 1921, resulting in a Nobel Prize, was considered one of the greatest scientific achievements of the 20th century. The development of purified animal insulins in the late 1970s, followed by human insulin in the early 1980s, resulted in dramatic reductions in allergic reactions and lipoatrophy. Introduction of the first generation of insulin analogs, insulin lispro in the mid-1990s followed by insulin glargine in the early 2000s, was an important advance for the treatment of type 1 diabetes. 54 We review the next generation of insulin analogs here. Table 4 provides details on available insulins.
Pharmacokinetics of commonly used insulin preparations
Ultra-long acting basal insulins
Insulin degludec was developed with the intention of improving the duration of action and achieving a flatter profile compared with the original long acting insulin analogs, insulin glargine and insulin detemir. Its duration of action of 42 hours at steady state means that the profile is generally flat without significant day-to-day variability, resulting in less hypoglycemia compared with U-100 glargine. 39 55
When U-100 insulin glargine is concentrated threefold, its action is prolonged. 56 U-300 glargine has a different kinetic profile and is delivered in one third of the volume of U-100 glargine, with longer and flatter effects. The smaller volume of U-300 glargine results in slower and more gradual release of insulin monomers owing to reduced surface area in the subcutaneous space. 57 U-300 glargine also results in lesser hypoglycemia compared with U-100 glargine. 58
Ultra-rapid acting prandial insulins
Rapid acting insulin analogs include insulin lispro, aspart, and glulisine. With availability of insulin lispro, the hope was for a prandial insulin that better matched food absorption. However, these newer insulins are too slow to control the glucose spike seen with ingestion of a high carbohydrate load, leading to the development of insulins with even faster onset of action.
The first available ultra-rapid prandial insulin was fast acting insulin aspart. This insulin has an onset of appearance approximately twice as fast (~5 min earlier) as insulin aspart, whereas dose-concentration and dose-response relations are comparable between the two insulins ( table 4 ). 59 In adults with type 1 diabetes, mealtime and post-meal fast acting aspart led to non-inferior glycemic control compared with mealtime aspart, in combination with basal insulin. 60 Mean HbA 1c was 7.3%, 7.3%, and 7.4% in the mealtime faster aspart, mealtime aspart, and post‐meal faster aspart arms, respectively (P<0.001 for non-inferiority).
Insulin lispro-aabc is the second ultra-rapid prandial insulin. In early kinetic studies, insulin lispro-aabc appeared in the serum five minutes faster with 6.4-fold greater exposure in the first 15 minutes compared with insulin lispro. 61 The duration of exposure of the insulin concentrations in this study was 51 minutes faster with lispro-aabc. Overall insulin exposure was similar between the two groups. Clinically, lispro-aabc is non-inferior to insulin lispro, but postprandial hyperglycemia is lower with the faster acting analog. 62 Lispro-aabc given at mealtime resulted in greater improvement in post-prandial glucose (two hour post-prandial glucose −31.1 mg/dL, 95% confidence interval −41.0 to −21.2; P<0.001).
Both ultra-rapid acting insulins can be used in insulin pumps. Lispro-aabc tends to have more insertion site reactions than insulin lispro. 63 A meta-analysis including nine studies and 1156 participants reported increased infusion set changes on rapid acting insulin analogs (odds ratio 1.60, 95% confidence interval 1.26 to 2.03). 64
Pulmonary inhaled insulin
The quickest acting insulin is pulmonary inhaled insulin, with an onset of action of 12 minutes and a duration of 1.5-3 hours. 65 When used with postprandial supplemental dosing, glucose control is improved without an increase in hypoglycemia. 66
Insulin delivery systems
Approved automated insulin delivery systems.
CGM systems and insulin pumps have shown improvement in glycemic control and decreased risk of severe hypoglycemia compared with use of self-monitoring of blood glucose and multiple daily insulin injections in type 1 diabetes. 67 68 69 Using CGM and insulin pump together (referred to as sensor augmented pump therapy) only modestly improves HbA 1c in patients who have high sensor wear time, 70 71 but the management burden of diabetes does not decrease as frequent user input is necessary. Thus emerged the concept of glucose responsive automated insulin delivery (AID), in which data from CGM can inform and allow adjustment of insulin delivery.
In the past decade, exponential improvements in CGM technologies and refined insulin dosing pump algorithms have led to the development of AID systems that allow for minimization of insulin delivery burden. The early AID systems reduced hypoglycemia risk by automatically suspending insulin delivery when glucose concentrations dropped to below a pre-specified threshold but did not account for high glucose concentrations. More complex algorithms adjusting insulin delivery up and down automatically in response to real time sensor glucose concentrations now allow close replication of normal endocrine pancreatic physiology.
AID systems (also called closed loop or artificial pancreas systems) include three components—an insulin pump that continuously delivers rapid acting insulin, a continuous glucose sensor that measures interstitial fluid glucose at frequent intervals, and a control algorithm that continuously adjusts insulin delivery that resides in the insulin pump or a smartphone application or handheld device ( fig 4 ). All AID systems that are available today are referred to as “hybrid” closed loop (HCL) systems, as users are required to manually enter prandial insulin boluses and signal exercise, but insulin delivery is automated at night time and between meals. AID systems, regardless of the type used, have shown benefit in glycemic control and cost effectiveness, improve quality of life by improving sleep quality, and decrease anxiety and diabetes burden in adults and children. 72 73 74 Limitations to today’s HCL systems are primarily related to pharmacokinetics and pharmacodynamics of available analog insulins and accuracy of CGM in extremes of blood glucose values. The iLet bionic pancreas, cleared by the US Food and Drug Administration (FDA) in May 2023, is an AID system that determines all therapeutic insulin doses for an individual on the basis of body weight, eliminating the need for calculation of basal rates, insulin to carbohydrate ratios, blood glucose corrections, and bolus dose. The control algorithms adapt continuously and autonomously to the individual’s insulin needs. 38 Table 5 lists available AID systems.

Schematic of closed loop insulin pump technology. The continuous glucose monitor senses interstitial glucose concentrations and sends the information via Bluetooth to a control algorithm hosted on an insulin pump (or smartphone). The algorithm calculates the amount of insulin required, and the insulin pump delivers rapid acting insulin subcutaneously
Comparison of commercially available hybrid closed loop systems 75
Unapproved systems
Do-it-yourself (DIY) closed loop systems—DIY open artificial pancreas systems—have been developed by people with type 1 diabetes with the goal of self-adjusting insulin by modifying their individually owned devices. 76 These systems are built by the individual using an open source code widely available to anyone with compatible medical devices who is willing and able to build their own system. DIY systems are used by several thousand people across the globe but are not approved by regulatory bodies; they are patient-driven and considered “off-label” use of technology with the patient assuming full responsibility for their use. Clinicians caring for these patients should ensure basic diabetes skills, including pump site maintenance, a knowledge of how the chosen system works, and knowing when to switch to “manual mode” for patients using an artificial pancreas system of any kind. 76 The small body of studies on DIY looping suggests improvement in HbA 1c , increased time in range, decreased hypoglycemia and glucose variability, improvement in night time blood glucose concentrations, and reduced mental burden of diabetes management. 77 78 79 Although actively prescribing or initiating these options is not recommended, these patients should be supported by clinical teams; insulin prescription should not be withheld, and, if initiated by the patient, unregulated DIY options should be openly discussed to ensure open and transparent relationships. 78
In January 2023, the US FDA cleared the Tidepool Loop app, a DIY AID system. This software will connect the CGM, insulin pump, and Loop algorithm, but no RCTs using this method are available.
β cell replacement therapies
For patients with type 1 diabetes who meet specific clinical criteria, β cell replacement therapy using whole pancreas or pancreatic islet transplantation can be considered. Benefits of transplantation include immediate cessation of insulin therapy, attainment of euglycemia, and avoidance of hypoglycemia. Additional benefits include improved quality of life and stabilization of complications. 80 Chronic immunosuppression is needed to prevent graft rejection after transplantation.
Pancreas transplantation
Whole pancreas transplantation, first performed in 1966, involves complex abdominal surgery and lifelong immunosuppressive therapy and is limited by organ donor availability. Today, pancreas transplants are usually performed simultaneously using two organs from the same donor (simultaneous pancreas-kidney transplant (SPKT)), sequentially if the candidate has a living donor for renal transplantation (pancreas after kidney transplant (PAKT)) or on its own (pancreas transplantation alone). Most whole pancreas transplants are performed with kidney transplantation for end stage diabetic kidney disease. Pancreas graft survival at five years after SPKT is 80% and is superior to that with pancreas transplants alone (62%) or PAKT (67%). 81 Studies from large centers where SPKT is performed show that recipients can expect metabolic improvements including amelioration of problematic hypoglycemia for at least five years. 81 The number of pancreas transplantations has steadily decreased in the past two decades.
Islet transplantation
Islet transplantation can be pursued in selected patients with type 1 diabetes marked by unawareness of hypoglycemia and severe hypoglycemic episodes, to help restore the α cell response critical for responding to hypoglycemia. 82 83 Islet transplantation involves donor pancreas procurement with subsequent steps to isolate, purify, culture, and infuse the islets. Multiple donors are needed to provide enough islet cells to overcome islet cell loss during transplantation. Survival of the islet grafts, limited donor supply, and lifelong need for immunosuppressant therapy remain some of the biggest challenges. 84 Islet transplantation remains experimental in the US and is offered in a few specialized centers in North America, some parts of Europe, and Australia. 85
Disease modifying treatments for β cell preservation
Therapies targeting T cells, B cells, and cytokines that find use in a variety of autoimmune diseases have also been applied to type 1 diabetes. The overarching goal of immune therapies in type 1 diabetes is to prevent or delay the loss of functional β cell mass. Studies thus far in early type 1 diabetes have not yet successfully shown reversal of loss of C peptide or maintenance of concentrations after diagnosis, although some have shown preservation or slowing of loss of β cells. This suggests that a critical time window of opportunity exists for starting treatment depending on the stage of type 1 diabetes ( fig 1 ).
Teplizumab is a humanized monoclonal antibody against the CD3 molecule on T cells; it is thought to modify CD8 positive T lymphocytes, key effector cells that mediate β cell death and preserves regulatory T cells. 86 Teplizumab, when administered to patients with new onset of type 1 diabetes, was unable to restore glycemia despite C peptide preservation. 87 However, in its phase II prevention study of early intervention in susceptible individuals (at least two positive autoantibodies and an abnormal oral glucose tolerance test at trial entry), a single course of teplizumab delayed progression to clinical type 1 diabetes by about two years ( table 2 ). 43 On the basis of these results, teplizumab received approval in the US for people at high risk of type 1 diabetes in November 2022. 88 A phase III trial (PROTECT; NCT03875729 ) to evaluate the efficacy and safety of teplizumab versus placebo in children and adolescents with new diagnosis of type 1 diabetes (within six weeks) is ongoing. 89
Thus far, targeting various components of the immune response has been attempted in early type 1 diabetes without any long term beneficial effects on C peptide preservation. Co-stimulation blockade using CTLA4-Ig abatacept, a fusion protein that interferes with co-stimulation needed in the early phases of T cell activation that occurs in type 1 diabetes, is being tested for efficacy in prevention of type 1 diabetes ( NCT01773707 ). 90 Similarly, several cytokine directed anti-inflammatory targets (interleukin 6 receptor, interleukin 1β, tumor necrosis factor ɑ) have not shown any benefit.
Non-immunomodulatory adjunctive therapies
Adjunctive therapies for type 1 diabetes have been long entertained owing to problems surrounding insulin delivery, adequacy of glycemic management, and side effects associated with insulin, especially weight gain and hypoglycemia. At least 50% of adults with type 1 diabetes are overweight or obese, presenting an unmet need for weight management in these people. Increased cardiovascular risk in these people despite good glycemic management presents additional challenges. Thus, use of adjuvant therapies may tackle these problems.
Metformin, by decreasing hepatic glucose production, could potentially decrease fasting glucose concentrations. 91 It has shown benefit in reducing insulin doses and possibly improving metabolic control in obese/overweight people with type 1 diabetes. A meta-analysis of 19 RCTs suggests short term improvement in HbA 1c that is not sustained after three months and is associated with higher incidence of gastrointestinal side effects. 92 No evidence shows that metformin decreases cardiovascular morbidity in type 1 diabetes. Therefore, owing to lack of conclusive benefit, addition of metformin to treatment regimens is not recommended in consensus guidelines.
Glucagon-like peptide receptor agonists
Endogenous GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion and enhances glucose induced insulin secretion, suppresses glucagon secretion, delays gastric emptying, and induces satiety. 93 GLP-1 promotes β cell proliferation and inhibits apoptosis, leading to expansion of β cell mass. GLP-1 secretion in patients with type 1 diabetes is similar to that seen in people without diabetes. Early RCTs of liraglutide in type 1 diabetes resulted in weight loss and modest lowering of HbA 1c ( table 2 ). 49 50 Liraglutide 1.8 mg in people with type 1 diabetes and higher body mass index decreased HbA 1c , weight, and insulin requirements with no increased hypoglycemia risk. 94 However, on the basis of results from a study of weekly exenatide that showed similar results, these effects may not be sustained. 51 A meta-analysis of 24 studies including 3377 participants showed that the average HbA 1c decrease from GLP-1 receptor agonists compared with placebo was highest for liraglutide 1.8 mg daily (−0.28%, 95% confidence interval −0.38% to−0.19%) and exenatide (−0.17%, −0.28% to 0.02%). The estimated weight loss from GLP-1 receptor agonists compared with placebo was −4.89 (−5.33 to−4.45) kg for liraglutide 1.8 mg and −4.06 (−5.33 to−2.79) kg for exenatide. 95 No increase in severe hypoglycemia was seen (odds ratio 0.67, 0.43 to 1.04) but therapy was associated with higher levels of nausea. GLP-1 receptor agonist use may be beneficial for weight loss and reducing insulin doses in a subset of patients with type 1 diabetes. GLP-1 receptor agonists are not a recommended treatment option in type 1 diabetes. Semaglutide is being studied in type 1 diabetes in two clinical trials ( NCT05819138 ; NCT05822609 ).
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter 2 (SGLT-2), a protein expressed in the proximal convoluted tubule of the kidney, reabsorbs filtered glucose; its inhibition prevents glucose reabsorption in the tubule and increases glucose excretion by the kidney. Notably, the action of these agents is independent of insulin, so this class of drugs has potential as adjunctive therapy for type 1 diabetes. Clinical trials have shown significant benefit in cardiovascular and renal outcomes in type 2 diabetes; therefore, significant interest exists for use in type 1 diabetes. Several available SGLT-2 inhibitors have been studied in type 1 diabetes and have shown promising results with evidence of decreased total daily insulin dosage, improvement in HbA 1c , lower rates of hypoglycemia, and decrease in body weight; however, these effects do not seem to be sustained at one year in clinical trials and seem to wane with time. Despite beneficial effects, increased incidence of diabetic ketoacidosis has been observed in all trials, is a major concern, and is persistent despite educational efforts. 96 97 98 Low dose empagliflozin (2.5 mg) has shown lower rates of diabetic ketoacidosis in clinical trials ( table 2 ). 47 Favorable risk profiles have been noted in Japan, the only market where SGLT-2 inhibitors are approved for adjunctive use in type 1 diabetes. 99 In the US, SGLT-2 inhibitors are approved for use in type 2 diabetes only. In Europe, although dapagliflozin was approved for use as adjunct therapy to insulin in adults with type 1 diabetes, the manufacturer voluntarily withdrew the indication for the drug in 2021. 100 Sotagliflozin is a dual SGLT-1 and SGLT-2 inhibitor that decreases renal glucose reabsorption through systemic inhibition of SGLT-2 and decreases glucose absorption in the proximal intestine by SGLT-1 inhibition, blunting and delaying postprandial hyperglycemia. 101 Studies of sotagliflozin in type 1 diabetes have shown sustained HbA 1c reduction, weight loss, lower insulin requirements, lesser hypoglycemia, and more diabetic ketoacidosis relative to placebo. 102 103 104 The drug received authorization in the EU for use in type 1 diabetes, but it is not marketed there. Although SGLT inhibitors are efficacious in type 1 diabetes management, the risk of diabetic ketoacidosis is a major limitation to widespread use of these agents.
Updates in acute complications of type 1 diabetes
Diabetic ketoacidosis.
Diabetic ketoacidosis is a serious and potentially fatal hyperglycemic emergency accompanied by significant rates of mortality and morbidity as well as high financial burden for healthcare systems and societies. In the past decade, increasing rates of diabetic ketoacidosis in adults have been observed in the US and Europe. 105 106 This may be related to changes in the definition of diabetic ketoacidosis, use of medications associated with higher risk, and admission of patients at lower risk. 107 In a US report of hospital admissions with diabetic ketoacidosis, 53% of those admitted were between the ages of 18 and 44, with higher rates in men than in women. 108 Overall, although mortality from diabetic ketoacidosis in developed countries remains low, rates have risen in people aged >60 and in those with coexisting life threatening illnesses. 109 110 Recurrent diabetic ketoacidosis is associated with a substantial mortality rate. 111 Frequency of diabetic ketoacidosis increases with higher HbA 1c concentrations and with lower socioeconomic status. 112 Common precipitating factors include newly diagnosed type 1 diabetes, infection, poor adherence to insulin, and an acute cardiovascular event. 109
Euglycemic diabetic ketoacidosis refers to the clinical picture of an increased anion gap metabolic acidosis, ketonemia, or significant ketonuria in a person with diabetes without significant glucose elevation. This can be seen with concomitant use of SGLT-2 inhibitors (currently not indicated in type 1 diabetes), heavy alcohol use, cocaine use, pancreatitis, sepsis, and chronic liver disease and in pregnancy 113 Treatment is similar to that for hyperglycemic diabetic ketoacidosis but can require earlier use and greater concentrations of a dextrose containing fluid for the insulin infusion in addition to 0.9% normal saline resuscitation fluid. 114
The diagnosis of diabetic ketoacidosis has evolved from a gluco-centric diagnosis to one requiring hyperketonemia. By definition, independent of blood glucose, a β-hydroxybutyrate concentration >3 mmol/L is required for diagnosis. 115 However, the use of this ketone for assessment of the severity of the diabetic ketoacidosis is controversial. 116 Bedside β-hydroxybutyrate testing during treatment is standard of care in many parts of the world (such as the UK) but not others (such as the US). Concerns have been raised about accuracy of bedside β-hydroxybutyrate meters, but this is related to concentrations above the threshold for diabetic ketoacidosis. 116
Goals for management of diabetic ketoacidosis include restoration of circulatory volume, correction of electrolyte imbalances, and treatment of hyperglycemia. Intravenous regular insulin infusion is the standard of care for treatment worldwide owing to rapidity of onset of action and rapid resolution of ketonemia and hyperglycemia. As hypoglycemia and hypokalemia are more common during treatment, insulin doses are now recommended to be reduced from 0.1 u/kg/h to 0.05 u/kg/h when glucose concentrations drop below 250 mg/dL or 14 mM. 115 Subcutaneous rapid acting insulin protocols have emerged as alternative treatments for mild to moderate diabetic ketoacidosis. 117 Such regimens seem to be safe and have the advantages of not requiring admission to intensive care, having lower rates of complications related to intravenous therapy, and requiring fewer resources. 117 118 Ketonemia and acidosis resolve within 24 hours in most people. 115 To prevent rebound hyperglycemia, the transition off an intravenous insulin drip must overlap subcutaneous insulin by at least two to four hours. 115
Hypoglycemia
Hypoglycemia, a common occurrence in people with type 1 diabetes, is a well appreciated effect of insulin treatment and occurs when blood glucose falls below the normal range. Increased susceptibility to hypoglycemia from exogenous insulin use in people with type 1 diabetes results from multiple factors, including imperfect subcutaneous insulin delivery tools, loss of glucagon within a few years of diagnosis, progressive impairment of the sympatho-adrenal response with repeated hypoglycemic episodes, and eventual development of impaired awareness. In 2017 the International Hypoglycemia Study Group developed guidance for definitions of hypoglycemia; on the basis of this, a glucose concentration of 3.0-3.9 mmol/L (54-70 mg/dL) was designated as level 1 hypoglycemia, signifying impending development of level 2 hypoglycemia—a glucose concentration <3 mmol/L (54 mg/dL). 119 120 At approximately 54 mg/dL, neuroglycopenic hypoglycemia symptoms, including vision and behavior changes, seizures, and loss of consciousness, begin to occur as a result of glucose deprivation of neurons in the central nervous system. This can eventually lead to cerebral dysfunction at concentrations <50 mg/dL. 121 Severe hypoglycemia (level 3), denoting severe cognitive and/or physical impairment and needing external assistance for recovery, is a common reason for emergency department visits and is more likely to occur in people with lower socioeconomic status and with the longest duration of diabetes. 112 Prevalence of self-reported severe hypoglycemia is very high according to a global population study that included more than 8000 people with type 1 diabetes. 122 Severe hypoglycemia occurred commonly in younger people with suboptimal glycemia according to a large electronic health record database study in the US. 123 Self- reported severe hypoglycemia is associated with a 3.4-fold increase in mortality. 124 125
Acute consequences of hypoglycemia include impaired cognitive function, temporary focal deficits including stroke-like symptoms, and memory deficits. 126 Cardiovascular effects including tachycardia, arrhythmias, QT prolongation, and bradycardia can occur. 127 Hypoglycemia can impair many activities of daily living, including motor vehicle safety. 128 In a survey of adults with type 1 diabetes who drive a vehicle at least once a week, 72% of respondents reported having hypoglycemia while driving, with around 5% reporting a motor vehicle accident due to hypoglycemia in the previous two years. 129 This contributes to the stress and fear that many patients face while grappling with the difficulties of ongoing hypoglycemia. 130
Glucagon is highly efficacious for the primary treatment of severe hypoglycemia when a patient is unable to ingest carbohydrate safely, but it is unfortunately under-prescribed and underused. 131 132 Availability of nasal, ready to inject, and shelf-stable liquid glucagon formulations have superseded the need for reconstituting older injectable glucagon preparations before administration and are now preferred. 133 134 Real time CGM studies have shown a decreased hypoglycemic exposure in people with impaired awareness without a change in HbA 1c . 34 135 136 137 138 CGM has shown benefit in decreasing hypoglycemia across the lifespan, including in teens, young adults, and older people. 36 139 Although CGM reduces the burden of hypoglycemia including severe hypoglycemia, it does not eliminate it; overall, such severe level 3 hypoglycemia rates in clinical trials are very low and hard to decipher in the real world. HCL insulin delivery systems integrated with CGM have been shown to decrease hypoglycemia. Among available rapid acting insulins, ultra-rapid acting lispro (lispro-aabc) seems to be associated with less frequent hypoglycemia in type 1 diabetes. 140 141
As prevention of hypoglycemia is a crucial aspect of diabetes management, formal training programs to increase awareness and education on avoidance of hypoglycemia, such as the UK’s Dose Adjustment for Normal Eating (DAFNE), have been developed. 142 143 This program has shown fewer severe hypoglycemia (mean 1.7 (standard deviation 8.5) episodes per person per year before training to 0.6 (3.7) episodes one year after training) and restoration of recognition of hypoglycemia in 43% of people reporting unawareness. Clinically relevant anxiety and depression fell from 24.4% to 18.0% and from 20.9% to 15.5%, respectively. A structured education program with cognitive and psychotherapeutic aspects for changing hypoglycemia related behaviors, called the Hypoglycemia Awareness Restoration Program despite optimized self-care (HARPdoc), showed a positive effect on changing unhelpful beliefs around hypoglycemia and improved diabetes related and general distress and anxiety scores. 144
Management in under-resourced settings
According to a recent estimate from the International Diabetes Federation, 1.8 million people with type 1 diabetes live in low and middle income countries (LMICs). 2 In many LMICs, the actual burden of type 1 diabetes remains unknown and material resources needed to manage type 1 diabetes are lacking. 145 146 Health systems in these settings are underequipped to tackle the complex chronic disease that is type 1 diabetes. Few diabetes and endocrinology specialist physicians are available owing to lack of specific postgraduate training programs in many LMICs; general practitioners with little to no clinical experience in managing type 1 diabetes care for these patients. 146 This, along with poor availability and affordability of insulin and lack of access to technology, results in high mortality rates. 147 148 149 In developed nations, low socioeconomic status is associated with higher levels of mortality and morbidity for adults with type 1 diabetes despite access to a universal healthcare system. 150 Although global governments have committed to universal health coverage and therefore widespread availability of insulin, it remains very far from realization in most LMICs. 151
Access to technology is patchy and varies globally. In the UST1DX, CGM use was least in the lowest fifth of socioeconomic status. 152 Even where technology is available, successful engagement does not always occur. 153 In a US cohort, lower CGM use was seen in non-Hispanic Black children owing to lower rates of device initiation and higher rates of discontinuation. 154 In many LMICs, blood glucose testing strips are not readily available and cost more than insulin. 151 In resource limited settings, where even diagnosis, basic treatments including insulin, syringes, and diabetes education are limited, use of CGM adds additional burden to patients. Need for support services and the time/resources needed to download and interpret data are limiting factors from a clinician’s perspective. Current rates of CGM use in many LMICs are unknown.
Inequities in the availability of and access to certain insulin formulations continue to plague diabetes care. 155 In developed countries such as the US, rising costs have led to insulin rationing by around 25% of people with type 1 diabetes. 156 LMICs have similar trends while also remaining burdened by disproportionate mortality and complications from type 1 diabetes. 155 157 With the inclusion of long acting insulin analogs in the World Health Organization’s Model List of Essential Medicines in 2021, hope has arisen that these will be included as standard of care across the world. 158 In the past, the pricing of long acting analogs has limited their use in resource poor settings 159 ; however, their inclusion in WHO’s list was a major step in improving their affordability. 158 With the introduction of lower cost long acting insulin biosimilars, improved access to these worldwide in the future can be anticipated. 160
Making insulin available is not enough on its own to improve the prognosis for patients with diabetes in resource poor settings. 161 Improved healthcare infrastructure, better availability of diabetes supplies, and trained personnel are all critical to improving type 1 diabetes care in LMICs. 161 Despite awareness of limitations and barriers, a clear understanding of how to implement management strategies in these settings is still lacking. The Global Diabetes Compact was launched in 2021 with the goal of increasing access to treatment and improving outcomes for people with diabetes across the globe. 162
Emerging technologies and treatments
Monitoring systems.
The ability to measure urinary or more recently blood ketone concentrations is an integral part of self-management of type 1 diabetes, especially during acute illness, intermittent fasting, and religious fasts to prevent diabetic ketoacidosis. 163 Many people with type 1 diabetes do not adhere to urine or blood ketone testing, which likely results in unnecessary episodes of diabetic ketoacidosis. 164 Noting that blood and urine ketone testing is not widely available in all countries and settings is important. 1 Regular assessment of patients’ access to ketone testing (blood or urine) is critical for all clinicians. Euglycemic diabetic ketoacidosis in type 1 diabetes is a particular problem with concomitant use of SGLT-2 inhibitors; for this reason, these agents are not approved for use in these patients. For sick day management (and possibly for the future use of SGLT-2 inhibitors in people with type 1 diabetes), it is hoped that continuous ketone monitoring (CKM) can mitigate the risks of diabetic ketoacidosis. 165 Like CGM, the initial CKM device measures interstitial fluid β-hydroxybutyrate instead of glucose. CKM use becomes important in conjunction with a hybrid closed loop insulin pump system and added SGLT-2 inhibitor therapy, where insulin interruptions are common and hyperketonemia is frequent. 166
Perhaps the greatest technological challenge to date has been the development of non-invasive glucose monitoring. Numerous attempts have been made using strategies including optics, microwave, and electrochemistry. 167 Lack of success to date has resulted in healthy skepticism from the medical community. 168 However, active interest in the development of non-invasive technology with either interstitial or blood glucose remains.
Insulin and delivery systems
In the immediate future, two weekly basal insulins, insulin icodec and basal insulin Fc, may become available. 169 Studies of insulin icodec in type 1 diabetes are ongoing (ONWARDS 6; NCT04848480 ). How these insulins will be incorporated in management of type 1 diabetes is not yet clear.
Currently available AID systems use only a single hormone, insulin. Dual hormone AID systems incorporating glucagon are in development. 170 171 Barriers to the use of dual hormone systems include the need for a second chamber in the pump, a lack of stable glucagon formulations approved for long term subcutaneous delivery, lack of demonstrated long term safety, and gastrointestinal side effects from glucagon use. 74 Similarly, co-formulations of insulin and amylin (a hormone co-secreted with insulin and deficient in people with type 1 diabetes) are in development. 172
Immunotherapy for type 1 diabetes
As our understanding of the immunology of type 1 diabetes expands, development of the next generation of immunotherapies is under active pursuit. Antigen specific therapies, peptide immunotherapy, immune tolerance using DNA vaccination, and regulatory T cell based adoptive transfer targeting β cell senescence are all future opportunities for drug development. Combining immunotherapies with metabolic therapies such as GLP-1 receptor agonists to help to improve β cell mass is being actively investigated.
The quest for β cell replacement methods is ongoing. Transplantation of stem cell derived islets offers promise for personalized regenerative therapies as a potentially curative method that does away with the need for donor tissue. Since the first in vivo model of glucose responsive β cells derived from human embryonic stem cells, 173 different approaches have been attempted. Mesenchymal stromal cell treatment and autologous hematopoietic stem cells in newly diagnosed type 1 diabetes may preserve β cell function without any safety signals. 174 175 176 Stem cell transplantation for type 1 diabetes remains investigational. Encapsulation, in which β cells are protected using a physical barrier to prevent immune attack and avoid lifelong immunosuppression, and gene therapy techniques using CRISPR technology also remain in early stages of investigation.
Until recently, no specific guidelines for management of type 1 diabetes existed and management guidance was combined with consensus statements developed for type 2 diabetes. Table 6 summarizes available guidance and statements from various societies. A consensus report for management of type 1 diabetes in adults by the ADA and European Association for the Study of Diabetes became available in 2021; it covers several topics of diagnosis and management of type 1 diabetes, including glucose monitoring, insulin therapy, and acute complications. Similarly, the National Institute for Health and Care Excellence also offers guidance on management of various aspects of type 1 diabetes. Consensus statements for use of CGM, insulin pump, and AID systems are also available.
Guidelines in type 1 diabetes
Conclusions
Type 1 diabetes is a complex chronic condition with increasing worldwide prevalence affecting several million people. Several successes in management of type 1 diabetes have occurred over the years from the serendipitous discovery of insulin in 1921 to blood glucose monitoring, insulin pumps, transplantation, and immunomodulation. The past two decades have seen advancements in diagnosis, treatment, and technology including development of analog insulins, CGM, and advanced insulin delivery systems. Although we have gained a broad understanding on many important aspects of type 1 diabetes, gaps still exist. Pivotal research continues targeting immune targets to prevent or delay onset of type 1 diabetes. Although insulin is likely the oldest of existing modern drugs, no low priced generic supply of insulin exists anywhere in the world. Management of type 1 diabetes in under resourced areas continues to be a multifaceted problem with social, cultural, and political barriers.
Glossary of abbreviations
ADA—American Diabetes Association
AID—automated insulin delivery
BGM—blood glucose monitoring
CGM—continuous glucose monitoring
CKM—continuous ketone monitoring
DCCT—Diabetes Control and Complications Trial
DIY—do-it-yourself
FDA—Food and Drug Administration
GADA—glutamic acid decarboxylase antibody
GLP-1—glucagon-like peptide 1
GRS—genetic risk scoring
HbA1c—glycated hemoglobin
HCL—hybrid closed loop
LADA—latent autoimmune diabetes of adults
LMIC—low and middle income country
PAKT—pancreas after kidney transplant
RCT—randomized controlled trial
SGLT-2—sodium-glucose cotransporter 2
SPKT—simultaneous pancreas-kidney transplant
Questions for future research
What future new technologies can be helpful in management of type 1 diabetes?
How can newer insulin delivery methods benefit people with type 1 diabetes?
What is the role of disease modifying treatments in prevention and delay of type 1 diabetes?
Is there a role for sodium-glucose co-transporter inhibitors or glucagon-like peptide 1 receptor angonists in the management of type 1 diabetes?
As the population with type 1 diabetes ages, how should management of these people be tailored?
How can we better serve people with type 1 diabetes who live in under-resourced settings with limited access to medications and technology?
How patients were involved in the creation of this manuscript
A person with lived experience of type 1 diabetes reviewed a draft of the manuscript and offered input on important aspects of their experience that should be included. This person is involved in large scale education and activism around type 1 diabetes. They offered their views on various aspects of type 1 diabetes, especially the use of adjuvant therapies and the burden of living with diabetes. This person also raised the importance of education of general practitioners on the various stages of type 1 diabetes and the management aspects. On the basis of this feedback, we have highlighted the burden of living with diabetes on a daily basis.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: SS and IBH contributed to the planning, drafting, and critical review of this manuscript. FNK contributed to the drafting of portions of the manuscript. All three authors are responsible for the overall content as guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SS has received an honorarium from Abbott Diabetes Care; IBH has received honorariums from Abbott Diabetes Care, Lifescan, embecta, and Hagar and research support from Dexcom and Insulet.
Provenance and peer review: Commissioned; externally peer reviewed.
- DeVries JH ,
- Hess-Fischl A ,
- Gregory GA ,
- Robinson TIG ,
- Linklater SE ,
- International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group
- Harding JL ,
- Wander PL ,
- Dabelea D ,
- Mayer-Davis EJ ,
- SEARCH for Diabetes in Youth Study
- Lawrence JM ,
- SEARCH for Diabetes in Youth Study Group
- Fazeli Farsani S ,
- Souverein PC ,
- van der Vorst MM ,
- Sutherland J ,
- Rokszin G ,
- Stahl-Pehe A ,
- Kamrath C ,
- Atkinson MA ,
- Bingley PJ ,
- Bonifacio E ,
- Leslie RD ,
- Evans-Molina C ,
- Freund-Brown J ,
- Thomas NJ ,
- Zimmet PZ ,
- Rowley MJ ,
- Knowles W ,
- Fourlanos S ,
- Greenbaum CJ ,
- ElSayed NA ,
- American Diabetes Association
- Miller RG ,
- Secrest AM ,
- Sharma RK ,
- Songer TJ ,
- McDonald T ,
- Greenfield JR
- Tridgell DM ,
- Spiekerman C ,
- Greenbaum CJ
- Glessner J ,
- Foster NC ,
- Miller KM ,
- Becker DJ ,
- Khawandanah J
- Pedrosa MR ,
- Franco DR ,
- Gieremek HW ,
- Nathan DM ,
- Diabetes Control and Complications Trial Research Group
- Klonoff DC ,
- Parkes JL ,
- Kovatchev BP ,
- Raghinaru D ,
- iDCL Trial Research Group
- Riddlesworth T ,
- DIAMOND Study Group
- Leelarathna L ,
- Neupane S ,
- FLASH-UK Trial Study Group
- Polonsky W ,
- Hirsch IB ,
- Pratley RE ,
- Kanapka LG ,
- Rickels MR ,
- Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group
- Tauschmann M ,
- APCam11 Consortium
- Russell SJ ,
- Damiano ER ,
- Bionic Pancreas Research Group
- Bailey TS ,
- Group Information ,
- Bergenstal RM ,
- Dellva MA ,
- Mathieu C ,
- Herold KC ,
- Type 1 Diabetes TrialNet Study Group
- Libman IM ,
- DiMeglio LA ,
- T1D Exchange Clinic Network Metformin RCT Study Group
- Petrie JR ,
- Chaturvedi N ,
- REMOVAL Study Group
- Dandona P ,
- Phillip M ,
- DEPICT-1 Investigators
- Rosenstock J ,
- Marquard J ,
- Laffel LM ,
- Hemmingsson JU ,
- ADJUNCT ONE Investigators
- Pieber TR ,
- ADJUNCT TWO Investigators
- Reynolds J ,
- Battelino T ,
- Liljenquist D ,
- Becker RH ,
- Bergmann K ,
- Lehmann A ,
- Cheng AYY ,
- Stender-Petersen K ,
- Hövelmann U ,
- Carlson AL ,
- Komatsu M ,
- Coutant DE ,
- Stamati A ,
- Karagiannis T ,
- Christoforidis A
- Heinemann L ,
- Baughman R ,
- Akturk HK ,
- Snell-Bergeon JK ,
- Prabhu JN ,
- Seyed Ahmadi S ,
- Westman K ,
- Pivodic A ,
- Hermann JM ,
- Freiberg C ,
- DPV Initiative
- Dicembrini I ,
- Cosentino C ,
- Mannucci E ,
- Abelseth J ,
- Karageorgiou V ,
- Papaioannou TG ,
- Weisman A ,
- Cardinez M ,
- Kramer CK ,
- Asarani NAM ,
- Reynolds AN ,
- Elbalshy M ,
- Jennings P ,
- Burnside MJ ,
- Williman JA ,
- Fridell JA ,
- Stratta RJ ,
- Gruessner AC
- Robertson RP
- Shapiro AM ,
- Pepper AR ,
- Shapiro AMJ
- Walker JT ,
- Saunders DC ,
- Brissova M ,
- Gitelman SE ,
- Bluestone JA
- Hagopian W ,
- Ferry RJ Jr . ,
- Protégé Trial Investigators
- von Scholten BJ ,
- Kreiner FF ,
- Gough SCL ,
- von Herrath M
- Type 1 Diabetes TrialNet Abatacept Study Group
- Hardie DG ,
- Dejgaard TF ,
- Christiansen E ,
- ADJUNCT ONE and ADJUNCT TWO Investigators
- Yunasan E ,
- Peters AL ,
- Palanca A ,
- van Nes F ,
- Ampudia Blasco FJ ,
- Dobbins RL ,
- Greenway FL ,
- Zambrowicz BP ,
- Brodovicz K ,
- Soleymanlou N ,
- Wissinger E ,
- Juhaeri J ,
- Mayer-Davis EJ
- Dhatariya KK ,
- Glaser NS ,
- Umpierrez GE
- Ramphul K ,
- Umpierrez G ,
- Korytkowski M
- Weinstock RS ,
- T1D Exchange Clinic Network
- Eshkoli T ,
- Brandstaetter E ,
- Jotkowitz A
- Dhatariya KK
- Joint British Diabetes Societies for Inpatient Care
- Kilpatrick ES ,
- Butler AE ,
- Ostlundh L ,
- Koufakis T ,
- Agiostratidou G ,
- International Hypoglycaemia Study Group
- van de Ven KC ,
- Heerschap A ,
- van der Graaf M ,
- de Galan BE
- Alsifri S ,
- Aronson R ,
- HAT Investigator Group
- Pettus JH ,
- Shepherd L ,
- Van Houten HK ,
- Ziegenfuss JY ,
- Wermers RA ,
- Schernthaner G ,
- Anderson J ,
- Saunders AL ,
- Snell-Bergeon J ,
- Forlenza GP ,
- Bispham J ,
- Gabbay RA ,
- Pontiroli AE ,
- Tagliabue E
- T1D Exchange Intranasal Glucagon Investigators
- Freckmann G ,
- Ehrmann D ,
- El Laboudi A ,
- Spanudakis E ,
- Anantharaja S ,
- Peleckis AJ ,
- Dalton-Bakes C ,
- van Beers CA ,
- Kleijer SJ ,
- CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group ,
- Piras de Oliveira C ,
- Ribeiro A ,
- Chigutsa F ,
- Malecki MT ,
- Hopkins D ,
- Lawrence I ,
- Mansell P ,
- Stanton-Fay SH ,
- Hamilton K ,
- Chadwick PM ,
- DAFNEplus study group
- Goldsmith K ,
- Yudkin JS ,
- Buntinx F ,
- Mapatano MA ,
- De Clerck M ,
- Truyers C ,
- Chambers D ,
- O’Cathain A
- Klatman EL ,
- Auzanneau M ,
- Tanenbaum ML ,
- Iturralde E ,
- Lipman TH ,
- Bhutta ZA ,
- Pfiester E ,
- Thieffry A ,
- Ballhausen H ,
- Gajewska KA ,
- O’Donnell S
- Wareham NJ ,
- Joosse HJ ,
- Raposo JF ,
- de Courten M
- Hemmingsen B ,
- Abouhassan T ,
- Albanese-O’Neill A ,
- Jacobsen L ,
- Haller MJ ,
- Castorino K ,
- Lovblom LE ,
- Cardinez N ,
- Nguyen KT ,
- Andersen G ,
- Meiffren G ,
- Famulla S ,
- Castellanos LE ,
- Balliro CA ,
- Sherwood JS ,
- Tsoukas MA ,
- Bernier-Twardy S ,
- Martinson LA ,
- Carlsson PO ,
- Schwarcz E ,
- Korsgren O ,
- Oliveira MC ,
- Stracieri AB ,
- Buzzetti R ,
- Mauricio D ,
- McGibbon A ,
- Ingersoll K ,
- Tugwell B ,
- Diabetes Canada Clinical Practice Guidelines Expert Committee
- Fleming GA ,
- McCall AL ,
- Gianchandani R ,
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Perceptions of adults with type 1 diabetes toward diabetes-specific quality of life measures: a survey-based qualitative exploration
Affiliations.
- 1 School of Psychology, Deakin University, Geelong, Australia. [email protected].
- 2 The Australian Centre for Behavioural Research in Diabetes, Diabetes Victoria, Carlton, Australia. [email protected].
- 3 Institute for Health Transformation, Deakin University, Geelong, Australia. [email protected].
- 4 School of Psychology, Deakin University, Geelong, Australia.
- 5 The Australian Centre for Behavioural Research in Diabetes, Diabetes Victoria, Carlton, Australia.
- 6 Institute for Health Transformation, Deakin University, Geelong, Australia.
- 7 Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
- 8 School of Health Sciences, University of Surrey, Guildford, UK.
- 9 Atlantis Health UK Ltd, London, UK.
- 10 Sheffield Centre for Health and Related Research (SCHARR), Sheffield CTRU, Sheffield, UK.
- 11 School of Medicine, Dept of Oncology and Metabolism, University of Sheffield, Sheffield, UK.
- PMID: 39218951
- PMCID: PMC11367869
- DOI: 10.1186/s12955-024-02285-4
Background: Diabetes-specific quality of life (QoL) questionnaires are commonly used to assess the impact of diabetes and its management on an individual's quality of life. While several valid and reliable measures of diabetes-specific QoL exist, there is no consensus on which to use and in what setting. Furthermore, there is limited evidence of their acceptability to people with diabetes. Our aim was to explore perceptions of adults with type 1 diabetes (T1D) toward five diabetes-specific QoL measures.
Methods: Adults (aged 18 + years) with T1D living in Australia or the United Kingdom (UK) were eligible to take part in 'YourSAY: QoL', an online cross-sectional survey. Recruitment involved study promotion on diabetes-related websites and social media, as well as direct invitation of people with T1D via a hospital client list (UK only). In random order, participants completed five diabetes-specific QoL measures: Audit of Diabetes-Dependent Quality of Life (ADDQoL-19); Diabetes Care Profile: Social and Personal Factors subscale (DCP); DAWN Impact of Diabetes Profile (DIDP); Diabetes-Specific Quality of Life Scale: Burden Subscale (DSQoLS); Diabetes Quality of Life Questionnaire (Diabetes QOL-Q). They were invited to provide feedback on each questionnaire in the form of a brief free-text response. Responses were analysed using inductive, thematic template analysis.
Results: Of the N = 1,946 adults with T1D who completed the survey, 20% (UK: n = 216, Australia: n = 168) provided qualitative responses about ≥ 1 measure. All measures received both positive and negative feedback, across four themes: (1) clarity and ease of completion, e.g., difficulty isolating impact of diabetes, dislike of hypothetical questions, and preference for 'not applicable' response options; (2) relevance and comprehensiveness, e.g., inclusion of a wide range of aspects of life to improve personal relevance; (3) length and repetition, e.g., length to be balanced against respondent burden; (4) framing and tone, e.g., preference for respectful language and avoidance of extremes.
Conclusions: These findings suggest opportunities to improve the relevance and acceptability of existing diabetes-specific QoL measures, and offer considerations for developing new measures, which need to be better informed by the preferences of people living with diabetes.
Keywords: Diabetes; Outcome measurement; Quality of life.
© 2024. The Author(s).
PubMed Disclaimer
Conflict of interest statement
Jane Speight owns the copyright for ‘Diabetes QOL-Q’. All other authors declare no conflict of interests.
Participant flow and reasons for…
Participant flow and reasons for exclusion
- Speight J, Holmes-Truscott E, Hendrieckx C, Skovlund S, Cooke D. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med. 2020;37(3):483–92. 10.1111/dme.14196 - DOI - PubMed
- Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome - A review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26(4):315–27. 10.1111/j.1464-5491.2009.02682.x - DOI - PubMed
- Fisher L, Tang T, Polonsky W. Assessing quality of life in diabetes: I. A practical guide to selecting the best instruments and using them wisely. Diabetes Res Clin Pract. 2017;126:278–85. 10.1016/j.diabres.2016.10.018 - DOI - PubMed
- Tang TS, Yusuf FL, Polonsky WH, Fisher L. Assessing quality of life in diabetes: II–Deconstructing measures into a simple framework. Diabetes Res Clin Pract. 2017;126:286–302. 10.1016/j.diabres.2016.10.007 - DOI - PubMed
- Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med. 2002;19(1):1–11. 10.1046/j.1464-5491.2002.00650.x - DOI - PubMed
- Search in MeSH
Grants and funding
- RP-PG-0514-20013/NIHR
LinkOut - more resources
Full text sources.
- BioMed Central
- PubMed Central
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Genetics of type-1 diabetes
- Mini-Review
- Published: 02 September 2024
Cite this article

- Hiroshi Ikegami ORCID: orcid.org/0000-0001-8808-4605 1 , 2 &
- Shinsuke Noso 3
Type-1 diabetes is a multifactorial disease characterized by genetic and environmental factors that contribute to its development and progression. Despite progress in the management of type-1 diabetes, the final goal of curing the disease is yet to be achieved. To establish effective methods for the prevention, intervention, and cure of the disease, the molecular mechanisms and pathways involved in its development and progression should be clarified. One effective approach is to identify genes responsible for disease susceptibility and apply information obtained from the function of genes in disease etiology for the protection, intervention, and cure of type-1 diabetes. In this review, we discuss the genetic basis of type-1 diabetes, along with prospects for its prevention, intervention, and cure for type-1 diabetes.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Genetic approaches to the study of gene variants and their impact on the pathophysiology of type 2 diabetes.
Genetics of diabetes mellitus and diabetes complications

Physiology Insights
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Seino Y, Nanjo K, Tajima N,et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int. 2010;1:2–20.
Kawasaki E, Maruyama T, Imagawa A, et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. Diabetol Int. 2013;4:221–5.
Article Google Scholar
Imagawa A, Hanafusa T, Awata T, et al. Report on the committee of the Japan Diabetes Society on the Research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). Diabetol Int. 2012;3:179–83.
Shimada A, Kawasaki E, Abiru N, et al. New diagnostic criteria (2023) for slowly progressive type 1 diabetes (SPIDDM): Report from Committee on Type 1 Diabetes of the Japan Diabetes Society (English version). Diabetol Int. 2024;15:1–4.
Article PubMed Google Scholar
Patterson C, Guariguata L, Dahlquist G, et al. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:161–75.
Imagawa A, Hanafusa T. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Prac Endocrinol Metab. 2007;3:36–45.
Ikegami H, Ogihara T. Genetics of insulin-dependent diabetes mellitus. Endocrine J. 1996;43:605–11.
Article CAS Google Scholar
Ikegami H, Kawabata Y, Noso S, et al. Genetics of type 1 diabetes in Asian and Caucasian populations. Diab Res Clin Prac. 2007;77(Suppl 1):S116–21.
Matsuda A, Kuzuya T. Diabetic twins in Japan. Diabetes Res Clin Pract. 1994;24(Suppl):S63–7.
Hyttinen V, Kaprio J, Kinnunen L, et al. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52:1052–5.
Article CAS PubMed Google Scholar
Ikegami H, Makino S. The NOD mouse and its related strains. In: Sima AAF, Shafrir E, editors. Animal Models of Diabetes A Primer. Amsterdam: Harwood Academic Publishers; 2000. p. 43–61.
Google Scholar
Komeda K, Noda M, Terao K, et al. Establishment of two substrains, diabetes-prone and non-diabetic, from Long-Evans Tokushima Lean (LETL) rats. Endocr J. 1998;45:737–44.
Ikegami H, Makino S, Harada M, et al. The cataract Shionogi mouse, a sister strain of the non-obese diabetic mouse: similar class II but different class I gene products. Diabetologia. 1988;31:254–8.
Ikegami H, Yano N, Sato T, et al. Immunogenetics and immunopathogenesis of the NOD mouse. In: Eisenbarth GS, editor., et al., Immunotherapy of diabetes and selected autoimmune diseases. Boca Raton: CRC Press; 1989. p. 22–33.
Hattori M, Buse JB, Jackson RA, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–5.
Wicker LS, Miller BJ, Coker LZ, et al. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987;165:1639–54.
Ikegami H, Makino S. Genetic susceptibility to insulin-dependent diabetes mellitus: from the NOD mouse to man. In: Shafrir E, editor. Frontiers in Diabetes Research: Lessons from Animal Diabetes IV. London: Smith-Gordon; 1993. p. 39–50.
Ikegami H, Makino S, Ogihara T. Molecular genetics of insulin-dependent diabetes mellitus: analysis of congenic strains. In: Shafrir E, editor. Frontiers in Diabetes Research: Lessons from Animal Diabetes VI. Boston: Birkhauser; 1996. p. 33–46.
Chapter Google Scholar
Kawabata Y, Ikegami H, Awata T, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21.
Awata T, Kawasaki E, Ikegami H, et al. Insulin gene/IDDM2 locus in Japanese type 1 diabetes: contribution of class I alleles and influence of class I subdivision in susceptibility to type 1 diabetes. J Clin Endocrinol Metab. 2007;92:1791–5.
Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci USA. 1987;84:2435–9.
Article CAS PubMed PubMed Central Google Scholar
Ikegami H, Eisenbarth GS, Hattori M. Major histocompatibility complex-linked diabetogenic gene of the nonobese diabetic mouse: analysis of genomic DNA amplified by the polymerase chain reaction. J Clin Invest. 1990;85:18–24.
Ikegami H, Makino S, Yamato E, et al. Identification of a new susceptibility locus for insulin-dependent diabetes mellitus by ancestral haplotype congenic mapping. J Clin Invest. 1995;96:1936–42.
Inoue K, Ikegami H, Fujisawa T, et al. Allelic variation in class I K gene as candidate for second component of MHC-linked susceptibility to type 1 diabetes in NOD mouse. Diabetologia. 2004;47:739–47.
Hattori M, Yamato E, Itoh N, et al. Cutting edge: homologous recombination of the MHC class I K region defines new MHC-linked diabetogenic susceptibility gene(s) in nonobese diabetic mice. J Immunol. 1999;163:1721–4.
Awata T, Kuzuya T, Matsuda A, et al. Genetic analysis of HLA class II alleles and susceptibility to type 1 (insulin-dependent) diabetes mellitus in Japanese subjects. Diabetologia. 1992;35:419–24.
Ikegami H, Kawaguchi Y, Yamato E, et al. Analysis by the polymerase chain reaction of histocompatibility leucocyte antigen-DR9-linked susceptibility to insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1992;75:1381–5.
CAS PubMed Google Scholar
Kawabata Y, Ikegami H, Kawaguchi Y, et al. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002;51:545–51.
Fujisawa T, Ikegami H, Yamato E, et al. Class I HLA is associated with age-at-onset of IDDM, while class II HLA confers susceptibility to IDDM. Diabetologia. 1995;38:1494.
Kawabata Y, Ikegami H, Kawaguchi Y, et al. Age-related association of MHC class I chain-related gene A (MICA) with type I (insulin-dependent) diabetes mellitus. Hum Immunol. 2000;61:624.
Nejentsev S, Howson JM, Walker NM, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–92.
Julier C, Hyer RN, Davies J, et al. Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4-dependent diabetes susceptibility. Nature. 1991;354:155–9.
Lucassen AM, Julier C, Beressi JP, et al. Susceptibility to insulin dependent diabetes mellitus maps to a 4.1kb segment of DNA spanning the insulin gene and associated with VNTR. Nat Genet. 1993;4:305–10.
Kawaguchi Y, Ikegami H, Shen G-Q, et al. Insulin gene region contributes to genetic susceptibility to, but may not to low incidence of, insulin-dependent diabetes mellitus in Japanese. Biochem Biophys Res Commun. 1997;233:283–7.
Vafiadia P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–92.
Pugliese A, Zeller M, Fernandez A Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–6.
Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3.
Noso S, Kataoka K, Kawabata Y, et al. Insulin transactivator MafA regulates intra-thymic expression of insulin and affects susceptibility to type 1 diabetes. Diabetes. 2010;59:2579–87.
Noso S, Kawabata Y, Babaya N, et al. Association study of MAFA and MAFB, genes related to organ-specific autoimmunity, with susceptibility to type 1 diabetes in Japanese and Caucasian populations. J Genet Syndr Gene Ther. 2013;4:204.
Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type 1 diabetes. Nat Genet. 2004;36:337–8.
Ueda H, Howson JMM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11.
Lowe CE, Cooper JD, Brusko T, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39:1074–82.
Ikegami H, Awata T, Kawasaki E, et al. The association of CTLA4 polymorphism with type 1 diabetes is concentrated in patients complicated with autoimmune thyroid disease: a multi-center collaborative study in Japan. J Clin Endocrinol Metab. 2006;91:1087–92.
Kawasaki E, Awata T, Ikegami H, et al. Systematic search for single nucleotide polymorphisms in a lymphoid tyrosine phosphatase (PTPN22) gene: Association between promoter polymorphism and type 1 diabetes in Asian populations. Am J Med Genet. 2006;140:586–93.
Awata T, Kawasaki E, Tanaka S, et al. Association of type 1 diabetes with two loci on 12q13 and 16p13 and the influence coexisting thyroid autoimmunity in Japanese. J Clin Endocrinol Metab. 2009;94:231–5.
Kawasaki E, Awata T, Ikegami H, et al. Genetic association between the IL2RA and mode of onset of type 1 diabetes in the Japanese population. J Clin Endocrinol Metab. 2009;94:947–52.
Yamashita H, Awata T, Kawasaki E, et al. Analysis of the HLA and non-HLA susceptibility loci in Japanese type 1 diabetes. Diabetes Metab Res Rev. 2011;27:844–8.
Ikegami H, Noso S, Babaya N, et al. Genetic basis of type 1 diabetes: similarities and differences between East and West. Rev Diabet Stud. 2008;5:64–72.
Article PubMed PubMed Central Google Scholar
Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177(1):26–31.
All of Us Research Program Genomics Investigators. Genomic data in the All of Us Research Program. Nature. 2024;627:340-6. https://doi.org/10.1038/s41586-023-06957-x . Online ahead of print.
Davies JL, Kawaguchi Y, Bennett ST, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371:130–6.
Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–64.
Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–7.
Chiou J, Geusz RJ, Okino ML, et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature. 2021;594:398–402.
Robertson CC, Inshaw JRJ, Onengut-Gumuscu S, et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat Genet. 2021;53:962–71.
Todd JA, Mijovic C, Fletcher J, et al. Identification of susceptibility loci for insulin-dependent diabetes mellitus by trans-racial gene mapping. Nature. 1989;338:587–9.
Ikegami H, Noso S, Babaya N, et al. Genetics and pathogenesis of type 1 diabetes: prospects for prevention and intervention. J Diabetes Investig. 2011;2:415–20.
Kawabata Y, Nishida N, Awata T, et al. A genome-wide association study confirming a strong effect of HLA and identifying variants in CSAD/lnc-ITGB7–1 on chromosome 12q13.13 associated with susceptibility to fulminant type 1 diabetes. Diabetes. 2019;68:665–75.
Kawabata Y, Ikegami H. Genetics of fulminant type 1 diabetes. Diabetol Int. 2020;11:315–22.
Arany E, Strutt B, Romanus P, et al. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia. 2004;47:1831–7.
Lin S, Yang J, Wu G, et al. Inhibitory effects of taurine on STZ-induced apoptosis of pancreatic islet cells. Adv Exp Med Biol. 2013;775:287–97.
Nakatsuru Y, Murase-Mishiba B-T M, et al. Taurine improves glucose tolerance in STZ-induced insulin-deficient diabetic mice. Diabetol Int. 2018;9:234–42.
Noso S, Babaya N, Hiromine Y, et al. Metabolic signatures of β-cell destruction in type 1 diabetes. J Diabetes Investig. 2023;14:48–57.
Sun BB, Kurki MI, Foley CN, et al. Genetic associations of protein-coding variants in human disease. Nature. 2022;603:95–102.
Olson ND, Wagner J, Dwarshuis N, et al. Variant calling and benchmarking in an era of complete human genome sequences. Nat Rev Genet. 2023;24:464–83.
Kishi A, Kawabata Y, Ugi S, et al. The onset of diabetes in three out of four sisters: a Japanese family with type 1 diabetes. a case report. Endocr J. 2009;56:767–72.
Ina Y, Kawabata Y, Sakamoto R, et al. A rare HLA genotype in two siblings with type 1 diabetes in a Japanese family clustered with type 1 diabetes. J Diabetes Investig. 2017;8:762–5.
Ikegami H. Molecular genetics of type 1 diabetes. J Jpn Diabetes Soc. 2024;67:1–7.
Noso S, Hosomichi K, Babaya N, et al. Whole-exome sequencing in rare families identified novel genetic variants for familial type 1 diabetes. Diabetologia. 2017;60(Suppl1):S24–5.
Ikegami H, Babaya N, Noso S. Beta-cell failure in diabetes: common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J Diabetes Investig. 2021;12:1526–39.
Willyard C. Can autoimmune diseases be cured? Scientists see hope at last Nature. 2024;625:646–8.
CAS Google Scholar
Dolgin E. How a pioneering diabetes drug offers hope for preventing autoimmune disorders. Nature. 2023;614:404–6.
Uno S, Imagawa A, Kozawa J, et al. Complete loss of insulin secretion capacity in type 1A diabetes patients during long-term follow up. J Diabetes Investig. 2018;9:806–12.
Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–53.
Niwano F, Babaya N, Hiromine Y, et al. Glucose metabolism after pancreatectomy: opposite extremes between pancreaticoduodenectomy and distal pancreatectomy. J Clin Endocrinol Metab. 2021;106:e2203–14.
Niwano F, Babaya N, Hiromine Y, et al. Three-year observation of glucose metabolism after pancreaticoduodenectomy: A single-center prospective study in Japan. J Clin Endocrinol Metab. 2022;107:3362–9.
Imamura S, Niwano F, Babaya N, et al. High incidence of diabetes mellitus after distal pancreatectomy and its predictors: A long-term follow-up study. J Clin Endocrinol Metab. 2024;109:619-30. https://doi.org/10.1210/clinem/dgad634 .
Baumel-Alterzon S, Katz LS, Brill G, et al. Nrf2: the master and captain of beta cell fate. Trends Endocrinol Metab. 2021;32:7–19.
Meyerovich K, Ortis F, Allagnat F, et al. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J Mol Endocrinol. 2016;57:R1–17.
Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J-I. Pancreatic beta-cell-specific expression of thioredoxin, an antioxidative and anti-apoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med. 1998;188:1445–51.
Yamamoto M, Yamato E, Shu-Ichi T, Tashiro F, Ikegami H, Yodoi J, Miyazaki J. Transgenic Expression of Antioxidant Protein Thioredoxin in Pancreatic β Cells Prevents Progression of Type 2 Diabetes Mellitus. Antioxid Redox Sig. 2008;10:43–50.
Ikegami H, Fujisawa T, Ogihara T. Mouse models of type 1 and type 2 diabetes derived from the same closed colony: genetic susceptibility shared between two types of diabetes? ILAR J. 2004;45:267–76.
Dooley J, Tian L, Schonefeldt S, et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48:519–27.
Rutsch N, Chamberlain CE, Dixon W, et al. Diabetes with multiple autoimmune and inflammatory conditions linked to an activating SKAP2 mutation. Diabetes Care. 2021;44:1816–25.
Hebbar P, Nizam R, John SE, et al. Linkage analysis using whole exome sequencing data implicates SLC17A1, SLC17A3, TATDN2 and TMEM131L in type 1 diabetes in Kuwaiti families. Sci Rep. 2023;13:14978.
Download references
Author information
Authors and affiliations.
Professor Emeritus, Kindai University, Osaka-sayama, Japan
Hiroshi Ikegami
Director of Health Administration Center and Nikkei Clinic, Human Resources, Nikkei Inc. Osaka Head Office, Osaka, Japan
Department of Endocrinology, Metabolism and Diabetes, Faculty of Medicine, Kindai University, Osaka-sayama, Japan
Shinsuke Noso
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hiroshi Ikegami .
Ethics declarations
Conflict of interest.
Hiroshi Ikegami: lecture fees (Novo Nordisk Pharma, Sanofi, Sumitomo Pharma), scholarship donations (LifeScan Japan, Sumitomo Pharma).
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Ikegami, H., Noso, S. Genetics of type-1 diabetes. Diabetol Int (2024). https://doi.org/10.1007/s13340-024-00754-1
Download citation
Received : 13 March 2024
Accepted : 06 August 2024
Published : 02 September 2024
DOI : https://doi.org/10.1007/s13340-024-00754-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- My Events / Fundraisers
- Monthly Giving
- Vehicle Donation
- Workplace Giving
- Find a Fundraiser
- Ways to Fundraise
We are Breakthrough T1D
We’re here to make every day with type 1 diabetes better, and we won’t stop until the condition is a thing of the past.

Get resources + support
As we drive toward curing type 1 diabetes (T1D), we help make everyday life better for the people who face it.

Learn about the symptoms of type 1 diabetes and get answers to common questions about the condition

Newly diagnosed
Get the support and information you need to understand and manage type 1 diabetes

Life with T1D
What you need to know about navigating life with type 1 diabetes at all ages and stages

T1D management
Learn about checking blood sugar, administering insulin, counting carbs, and more

Breakthrough T1D is the leading global type 1 diabetes research and advocacy organization.
Get involved.
Advocate. Engage. Inspire. Participate. Volunteer. Help us accelerate breakthroughs for T1D!

Connect with the community
Connect with a supportive local + global T1D community
Power the movement
We’re creating a movement for the entire T1D community

Accelerating T1D breakthroughs
This is more than a moment—we’re creating a movement. We connect the brightest minds to help advance treatments, influence policy, and improve access to care for those all over the world who need it. Today, we are leading the way to more effective solutions. Tomorrow, we will make this condition a thing of the past.
Breakthrough T1D news + updates
T1D information and developments you need to know

Navigating the school year with type 1 diabetes: A call to support and action

Strength, struggle, and resilience: my mom’s journey with T1D

At ADA 2024, Breakthrough T1D-funded research takes center stage
Be the first to know about t1d news, events and more.
Sign up to receive updates from Breakthrough T1D
- First name *
- Last name *
- Comments This field is for validation purposes and should be left unchanged.
Join the movement to end T1D
Champion federal funding for T1D research. Inform health and regulatory policy. Improve lives.
Your privacy
We value your privacy. When you visit BreakthroughT1D.org (and our family of websites), we use cookies to process your personal data in order to customize content and improve your site experience, provide social media features, analyze our traffic, and personalize advertising. By choosing “I Agree”, you understand and agree to Breakthrough T1D's Privacy Policy
- Español ( Spanish )
- Open access
- Published: 02 September 2024
Perceptions of adults with type 1 diabetes toward diabetes-specific quality of life measures: a survey-based qualitative exploration
- Elizabeth Holmes-Truscott 1 , 2 , 3 ,
- Jasmine Schipp 1 , 2 , 3 , 4 ,
- Debbie D. Cooke 5 , 6 ,
- Christel Hendrieckx 1 , 2 , 3 ,
- Elizabeth J. Coates 7 ,
- Simon R. Heller 8 &
- Jane Speight 1 , 2 , 3
Health and Quality of Life Outcomes volume 22 , Article number: 70 ( 2024 ) Cite this article
Metrics details
Diabetes-specific quality of life (QoL) questionnaires are commonly used to assess the impact of diabetes and its management on an individual’s quality of life. While several valid and reliable measures of diabetes-specific QoL exist, there is no consensus on which to use and in what setting. Furthermore, there is limited evidence of their acceptability to people with diabetes. Our aim was to explore perceptions of adults with type 1 diabetes (T1D) toward five diabetes-specific QoL measures.
Adults (aged 18 + years) with T1D living in Australia or the United Kingdom (UK) were eligible to take part in ‘YourSAY: QoL’, an online cross-sectional survey. Recruitment involved study promotion on diabetes-related websites and social media, as well as direct invitation of people with T1D via a hospital client list (UK only). In random order, participants completed five diabetes-specific QoL measures: Audit of Diabetes-Dependent Quality of Life (ADDQoL-19); Diabetes Care Profile: Social and Personal Factors subscale (DCP); DAWN Impact of Diabetes Profile (DIDP); Diabetes-Specific Quality of Life Scale: Burden Subscale (DSQoLS); Diabetes Quality of Life Questionnaire (Diabetes QOL-Q). They were invited to provide feedback on each questionnaire in the form of a brief free-text response. Responses were analysed using inductive, thematic template analysis.
Of the N = 1,946 adults with T1D who completed the survey, 20% (UK: n = 216, Australia: n = 168) provided qualitative responses about ≥ 1 measure. All measures received both positive and negative feedback, across four themes: (1) clarity and ease of completion, e.g., difficulty isolating impact of diabetes, dislike of hypothetical questions, and preference for ‘not applicable’ response options; (2) relevance and comprehensiveness, e.g., inclusion of a wide range of aspects of life to improve personal relevance; (3) length and repetition, e.g., length to be balanced against respondent burden; (4) framing and tone, e.g., preference for respectful language and avoidance of extremes.
Conclusions
These findings suggest opportunities to improve the relevance and acceptability of existing diabetes-specific QoL measures, and offer considerations for developing new measures, which need to be better informed by the preferences of people living with diabetes.
Diabetes-specific quality of life (QoL) refers to an individual’s perception of the impact of diabetes on their QoL, or how diabetes affects aspects of life important to them [ 1 , 2 ]. Several diabetes-specific QoL assessment tools exist [ 2 , 3 , 4 , 5 ], with no consensus on which to use and in what setting. Likely relatedly, there has also been a lack of systematic QoL assessment (diabetes-specific or generic) in diabetes research and clinical practice [ 1 ]. In response, practical guidance and frameworks have been proposed to support appropriate diabetes-specific QoL questionnaire selection [ 1 , 3 , 6 ], including consideration of questionnaire aims, intended population, rigour of development process, psychometric properties, sensitivity to change, participant burden, content face validity, and acceptability among the intended audience. While considerable evidence for the psychometric properties of established diabetes-specific QoL questionnaires (across populations and linguistic translations) exists, responder perceptions toward, and acceptability of, questionnaire’s remains limited [ 3 , 4 , 7 ].
Involvement of the intended group in questionnaire design and refinement is considered a methodological necessity to assure content validity and acceptability [ 8 , 9 , 10 , 11 ]. However, the extent to which people with diabetes have traditionally been consulted in the development of diabetes-specific QoL measures varies (see Table 1 ), and few subsequent studies have examined respondent perceptions to inform questionnaire refinement or selection [ 7 , 12 , 13 ]. While some more recently developed questionnaires have involved such community involvement and piloting [ 14 ], questionnaires developed decades ago continue to be used most widely and whether they remain fit for purpose or are acceptable to contemporary study participants warrants consideration. Researchers and clinicians must rely on the typically sparse details reported within in scale development and psychometric testing publications, with questionnaire acceptability often indicated by response rate rather the respondent perceptions.
Previously, we reported quantitative findings of the ‘Your Self-management And You: Quality of Life’ (YourSAY: QoL) study, which was the first ‘head-to-head’ comparison of the psychometric properties and acceptability of five questionnaires designed to assess diabetes-specific QoL among adults with type 1 diabetes (T1D) living in Australia and the United Kingdom (UK) [ 7 ]. The findings suggested largely positive and consistent acceptability user ratings across the diabetes-specific QoL measures, as quantitatively derived by five study-specific single-items on a 5-point Likert scale. However, these published data provide no insights into the specific reasons for the largely positive views, nor explanation for respondents’ negative ratings. In addition to the user ratings, survey participants were invited to provide brief qualitative feedback. In combination with published development processes and psychometric evaluation, the preferences of people with T1D can inform recommendations for the selection of diabetes-specific QoL tools as well as the improvement of existing and novel questionnaires can be made.
Therefore, the current study explores the qualitative feedback collected in the YourSAY: QoL survey to understand what adults with T1D in Australia and the UK like and dislike about five contemporary and/or commonly used diabetes-specific QoL measures.
The YourSAY: QoL study was a cross-sectional survey administered online, using a pragmatic mixed-methods approach to explore questionnaire acceptability. Study methods have been described in detail elsewhere, and are included below [ 7 ]. For this substudy, a descriptive theoretical framework was employed with the aim of providing a comprehensive summary of participant questionnaire perceptions and preferences. This approach is well-suited to ‘thin’ data collected via survey free-text responses.
Participants and recruitment
Eligible participants for the overall survey were adults (aged 18 + years) with a self-reported diagnosis of T1D or type 2 diabetes (T2D), living in either Australia or the UK. Participants were recruited using convenience sampling through websites, e-newsletters/blogs and social media (Twitter, Facebook). In the UK only, a social media advertising company was contracted to promote study advertisements using Facebook, and 1,921 consenting adults with T1D under the care of Sheffield Teaching Hospitals NHS Trust were invited to take part (via letter or email). Potential participants were directed to an online survey hosted via Qualtrics™ (a secure online survey platform). Following informed consent and eligibility screening, eligible participants were directed to the survey proper.
The YourSAY: QoL survey was completed by N = 4166 participants (T1D: n = 1946, 47%). Inclusion criteria for the current analysis were self-reported T1D, and provision of qualitative feedback on at least one attempted diabetes-specific QoL questionnaires. Participant flow and reasons for exclusion are detailed in Fig. 1 . The current qualitative study reports on a subsample of n = 384 YourSAY: QoL participants.
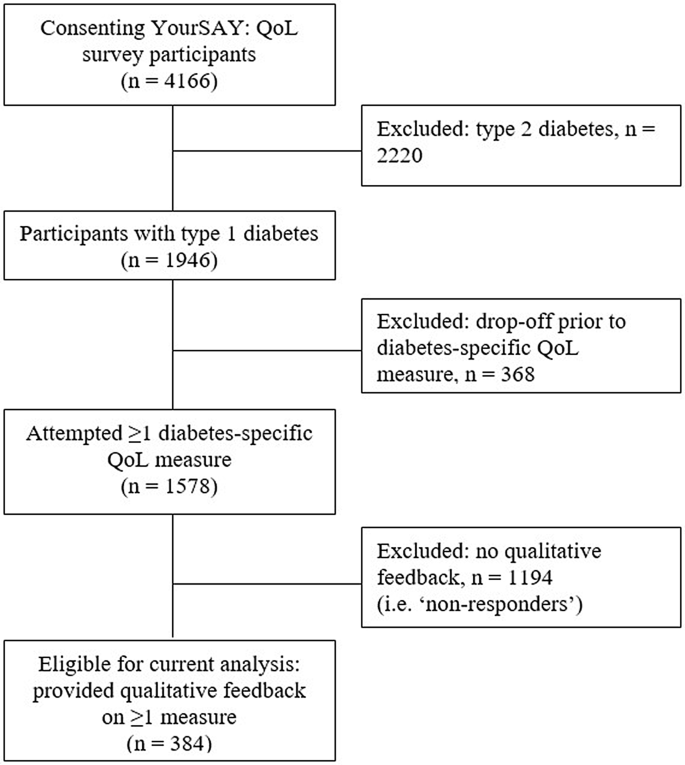
Participant flow and reasons for exclusion
Participants were invited to complete five diabetes-specific QoL measures (detailed in Table 1 ).The questionnaire selection process and psychometric analyses are reported elsewhere [ 7 ]. The five Diabetes-specific QoL measures were presented in random order to control for order effects, and optimise complete data per questionnaire in the case of early drop off. Following each questionnaire, participants were presented with five study-specific questions in which they were asked to rate ( 5-point scale: 1 = strongly disagree to 5 = strongly agree), the clarity, relevance, ease of completion, length, and comprehensiveness of the questionnaire (reported elsewhere) [ 7 ]. Participants were then asked the following free-text qualitative question: “Your feedback is important to us. Please feel free to comment below on anything you particularly liked or disliked about this questionnaire”.
Demographic (age, sex, location of residence, primary language) and self-reported clinical data (diabetes duration, primary treatment, number of diabetes-related complications) were also collected.
Data handling and analysis
Descriptive statistics for key demographic and clinical characteristics and differences between ‘responders’ (eligible final sample) and non-responders (participants with T1D who attempted ≥ 1 measure but provided no qualitative feedback) were calculated (IBM SPSS Statistics). Between group differences were assessed via Student’s t-tests for continuous variables and Pearson’s Chi-Square for categorical data.
Qualitative data were screened for invalid responses (e.g. “N/A”, “nil”) and uploaded to QSR NVivo for thematic template analysis, applying an inductive (data-driven) approach [ 22 ]. In template analysis, a coding template (which summarises the (sub)themes in a meaningful order) is developed, refined and applied iteratively to the data. This approach is well-suited to vast but shallow survey data collection, as template analysis does not require distinction between descriptive and interpretive themes. Following familiarisation with the data, J.Sc proposed an initial coding template, which was iteratively reviewed and refined following coding application by JSc and E.H-T, as well as discussion with J.Sp. Minimal coding disagreement was identified, discussed and resolved between authors. The final framework was applied to the remaining responses by J.Sc. Names and descriptions of the final themes and subthemes were agreed among all authors, and example quotes reviewed to ensure they represented the data adequately and reflected the study aims.
J.Sc, an undergraduate researcher at the time of analysis, led qualitative data analysis, reflecting on data initially without pre-conceived assumptions about measures, and discussing findings with the remaining authors (expertise: health psychology, health services, clinical diabetes) who drew on their deep understanding [ 1 , 2 ], and prior application of the assessed tools (including contributions to their development [ 21 ], refinement, [ 18 ] or English translation [ 20 ]) in their interpretation of findings. Representation of both positive and negative feedback for each measure was prioritised to challenge any predefined ideas about preferred measures.
Characteristics of the eligible sample (respondents, n = 384), and those who attempted the diabetes-specific QoL questionnaires but did not provide qualitative feedback (non-respondents, n = 1194), are shown in Table 2 .
Most respondents attempted all five diabetes-specific QoL measures of interest ( n = 344, 90%) and provided qualitative feedback on one questionnaire ( n = 220, 57%), most commonly for the DSQoLS (see Table 3 ). Across the five measures, a total of 711 qualitative responses were provided. A minority of participant feedback included general comments (e.g. all five questionnaires were perceived by some as “good”, “fine”, “liked”, or described as “enjoyable” and “interesting”) with no further detail. Specific feedback was organised within four main themes: (1) clarity and ease of completion; (2) relevance and comprehensiveness; (3) length and repetition, and (4) preferences and impact of questionnaire wording and tone. Overall themes and sub-themes are described below, and quotes relevant to the five questionnaires are shown in Table 3 .
Questionnaire clarity and ease of completion
In addition to general endorsements that questionnaires are “simple”, “easy”, and “straightforward”, participants provided specific feedback relating to the clarity of instructions and questionnaire wording; suitability of response options, and difficulty isolating the impact of diabetes on QoL.
Instructions and question wording
Differing views were offered about whether questionnaire instructions or questions were clear and easy to understand, as well as preferred questionnaire attributes. For instance, some participants praised the inclusion of examples in the Diabetes QOL-Q (‘I can go out or socialise as I would like, e.g., cinema, concerts…’), suggesting that it helped to clarify what is being asked, while another participant reported the inclusion of such examples as “patronising”.
Consistently, respondents indicated that the if/then wording used in the ADDQoL-19 was “confusing” and questions containing double negatives (i.e. item 17: “If I did not have diabetes, I would have to depend on others when I do not want to”) were “illogical” and “incomprehensible”. They also indicated that the broad question wording that encompassed diverse situations made it difficult to answer the questions. For example, item 3 of the DIDP incorporates three separate concepts (i.e. ‘relationship with your family, friends and peers’) and item 8 of the ADDQoL-19 combines current experience of or wish for a ‘close personal relationship’ within a single question. Participants also found overly-specific wording did not capture their actual experience. For example, two DCP items assess the impact of diabetes on food intake, but not “when I would like” to eat. One respondent provided feedback on the seventh item of the modified DIDP (‘Your freedom to eat as you wish’), reporting that this question stood out and was “much more specific than the other[s]”.
For the DCP and DSQoLS, participants also indicated that the timeframe referred to in the instructions (e.g. “in the past 4 weeks”, “in the past year”) was confusing, “easily forgotten”, or “too long”.
Response options
There was mixed feedback regarding the preferred number of response options and phrasing. Some participants reported liking the bi-directional response scale used in the DIDP, while others stated that they found it confusing or that it did not account for the potential combined positive and negative impacts of diabetes on particular aspects of life, such as physical health. In general, participants favoured inclusion of a “not applicable” response option, and some reported that this option was missing from the DCP and DSQoLS. Across measures, several participants indicated a desire to explain the underlying reasons for their response and participants positively reviewed the inclusion of an open-ended question inviting a free-text response in the ADDQoL-19.
Difficulty assessing the impact of diabetes
In rating the impact of diabetes on a particular aspect of life, the ADDQoL-19 asks participants to imagine their life without diabetes (e.g. ‘If I did not have diabetes, my quality of life would be…’). Though some participants reported that the opportunity to reflect on their life without diabetes was “an interesting concept”, others disliked this hypothetical phrasing and found the questions “impossible” to answer. While the other four measures of interest do not instruct participants to compare or rate their QoL against a life without diabetes, some participants reported difficultly responding as it’s “the only life I know” and that they may have over- or underestimated the impact of diabetes on their QoL. Participants also reported difficulty isolating diabetes from the impact of other life factors (e.g., other health condition, responsibilities, financial challenges). Some indicated a preference for a generic measure, which would allow participants to rate their QoL overall.
Questionnaire comprehensiveness and relevance
Where participants reported questionnaires as irrelevant, some indicated an inability to identify with the questionnaire, or that the questionnaire assumed a view of diabetes that did not reflect (their) reality. The lengthy DSQoLS (57 items) had the most references discussing perceptions of its (ir)relevance, while feedback on the shorter DIDP (7 items) indicated that it was “simplistic”.
Relevance of specific aspects of life and perceived omissions
Conflicting feedback was identified regarding the relevance of measured domains, particularly related to the impact of diabetes on finances. More typically, UK participants perceived such questions as irrelevant and suggested their removal, while Australian respondents endorsed such questions as relevant. Other specific questionnaire items reported as relevant included future health and development of diabetes complications (DSQoLS); emotional well-being (DIDP and DSQoLS, ); body image and family relationships (Diabetes QOL-Q). In contrast, irrelevant items commonly related to romantic partners and intimacy (DSQoLS, ADDQoL-19); driving (Diabetes QOL-Q); eating as you wish (ADDQoL-19); treatment modality-specific questions (DSQoLS), and experience of hypoglycaemia (DSQoLS).
Table 4 lists topics, or aspects of life, which participants perceived as missing from a questionnaire or not adequately assessed. Across questionnaires, commonly perceived omissions related to the impact of diabetes on mental / emotional health, and on finances, as well as the impact of diabetes management activities (e.g. insulin administration modality; diet; glucose monitoring) and extreme glucose levels on overall QoL. The Diabetes QOL-Q had the fewest references to omissions overall ( n = 22), while the DSQoLS had the most reported omissions ( n = 69), of which most referred to the impact of hyperglycaemia.
Questionnaire length and repetition
Related to, but discrete from comprehensiveness, participants commented on the length of measures, and were divided in their preference for brevity versus breadth. For example, the 7-item DIDP was described both as “brief and direct” and “so brief it was almost offensive”. Similarly, the 57-item DSQoLS was reported by some to be “too long” and by others as allowing for a detailed or comprehensive assessment (Theme 2). Item repetition was reported for the DSQoLS, to a lesser degree for the DCP and Diabetes QOL-Q and not at all for the ADDQoL-19 or the DIDP. Participants reported that repetitive questioning was “depressing”, overstated the relevance of certain topics/life aspects (i.e. the 11 DSQoLS items examining the impact of hypoglycaemia), and left them feeling more “worried”.
Questionnaire framing
Some respondents reported disliking the negative framing used by the ADDQoL-19, DCP and DSQoLS because they perceived it as placing a focus on the limitations of diabetes. In contrast, others appreciated the ADDQoL-19’s recognition of the “negative aspects of life”. Feedback about the positively-framed Diabetes QOL-Q was similarly divided between those who liked the “more positive manner” and those who felt it painted a “falsely positive picture”. Participants also reflected on the specific words and phrases used, reporting that certain terms (e.g. ‘normal’ and ‘control’ in the Diabetes QOoL-Q; ‘burden’, ‘worry’, ‘bother’ in the DSQoLS) made them feel like a “victim” or implied that diabetes controlled them. In contrast, the language used in the ADDQoL-19 was described as “extremely respectful”.
Consistent with quantitative user rating assessment [ 7 ], study findings suggest that there is no unequivocally favoured diabetes-specific QoL measure among adults with T1D from Australia and the UK. However, an acceptable measure needs to be easy to understand and complete; comprehensive and personally relevant; brief, without repetition; neither overly negative/nor positive; and adopting respectful language. Review of the questionnaires’ attributes (Table 1 ) suggests greatest alignment to reported preferences for the ADDQoL-19, DIDP, and Diabetes QOoL-Q (i.e. fewer reported omissions, greater perceived relevance, opportunity for personalisation, no/low repetition of domains, and/or neutrally worded). These three measures were also previously identified as having strongest psychometric performance among YourSAY: QOL participants with T1D [ 7 ]. Consideration and application of respondents’ preferences in the review of existing measures, or the development of new questionnaires, may further improve acceptability, respondent experience and data quality for future studies.
Careful consideration of questionnaire wording is needed to minimise cognitive load and improve acceptability [ 23 ]. For example, the ADDQoL-19 has been criticized for its unique use of hypothetical questioning [ 2 , 24 ], which is presumed to be cognitively demanding [ 24 , 25 ], and is not recommended [ 9 ]. While ADDQoL-19 developers suggest that such questioning results in a more realistic assessment of the impact of diabetes [ 26 ], the differential basis for hypothetical responses (e.g. some may recall a time pre-diabetes, others may draw on social comparisons) may impact data reliability. Interestingly, difficulty assessing the specific impact of diabetes due to a lack of comparison (i.e. life without diabetes) or inability to isolate its impacts (i.e. from other health conditions or life factors) was not unique to the ADDQoL-19, but reported across all measures. Relatedly, a preference for a more holistic measure of health-related or general QoL (e.g. the new EQ-HWB) [ 27 ] was reported by some participants, while for others, diabetes-specific questionnaires enabled a unique opportunity for reflection and acknowledgment of the challenges of diabetes. Further qualitative research might examine individual differences in how respondents interpret and complete condition-specific questionnaires.
Some participants reported difficultly completing questions that included double-barreled concepts (e.g. DIDP item: ‘your relationship with your family, friends and peers’) or were too broad (e.g. DIDP item: ‘your physical health’). Their separation into distinct items may be considered, though this has implications for scale brevity and could place too much emphasis on specific domains (as was reported for the DSQoLS regarding hypoglycaemia and emotional burden). Regardless, omission of important QoL domains was not more commonly reported for the brief DIDP (which higher-order type wording) and good concurrent validity between DIDP total scores and longer measures has been established [ 7 ]. Thus, the DIDP may be appropriate where the intention is to measure the overall impact of diabetes on QoL, and/or identify global domains for further assessment or clinical discussion. Regardless of item specificity, or consistency with theorised domains of diabetes-specific QoL [ 28 ], none of the examined measures in the current study were without perceived omissions of important life aspects (domains).
Determinants of QoL are subjective and, accordingly, it is argued that QoL assessment should be tailored to the aspects of life deemed most important to a given individual [ 29 ]. For example, several participants commented on the irrelevance of finances, while others expressly reported the importance or omission of this domain. The inclusion and rating of irrelevant or unimportant QoL domains, and the exclusion of other relevant domains, may reduce meaningful scoring and/or lead to misguided intervention. However, few QoL questionnaires allow for such personalised assessment [ 30 ]. In an effort to personalise QoL within a standardised approach, the ADDQoL-19 employs average weighted impact scores incorporating perceived domain importance within scoring, and three measures allow for non-applicable responses to all (DIDP, Diabetes QOL-Q) or certain (ADDQoL-19) domains, which was viewed favourably by participants. Though applicability is not synonymous with importance. The collection of qualitative data in companion with quantitatively assessed QoL might help bridge the divide between personalised and standardized assessment [ 31 ]. Participants reported appreciation of the ADDQoL-19 free-text question, and across other measures reported a desire to elaborate on their responses. Regardless of the measure selected, inclusion of a free-text question might be considered for the mutual benefit of examining survey acceptability, increasing insights into the experience of diabetes, and/or identifying areas for clinical discussion. It is important that ethical consideration is given to the intended use of such data, and its collection is justifiable.
Interestingly, negative feedback suggesting scale irrelevance, and omission of important life aspects more typically related to the considerably longer DSQoLS, which does not allow for ‘not applicable’ responses and is the only evaluated measure intended only for use among adults with T1D (i.e. not also for use in T2D). At previously noted [ 7 ], the DSQOLS item profile is dissimilar to other questionnaires assessed, with inclusion of items relating to diabetes management, symptoms, fear of hypoglycaemia, and emotional burden. In contrast, the ADDQoL-19, DIDP and Diabetes QOL-Q assess the impact of diabetes on more global aspects of life, which may be consistent across diabetes types, treatments and experiences. However, the current findings cannot speak to the perceptions of, nor preferred measure attributes among those with T2D. It is possible that planned future inspection of the acceptability and psychometric performance of relevant questionnaires among participants with T2D may highlight differential preferences and scale performance, suggesting the need for tailored questionnaire selection, revision, or development by diabetes type (as has been a recent focus in the assessment of diabetes distress [ 32 , 33 ]).
The overall strengths and limitations of the YourSAY: QoL study are detailed elsewhere [ 7 ]. A key strength of the current qualitative study is the survey method, which permitted feedback from a large sample, extending on prior quantitative exploration of questionnaire acceptability [ 7 ] and qualitative methods used to inform questionnaire development and/or debriefing. However, the inability to follow up participants and invite additional information is a limitation of the current approach. Further, a minority of participants provided qualitative feedback (likely due in part to the burdensome overall survey length which resulted in substantial participant attrition) and this group were found to be more highly educated and engaged and slightly less negatively impacted by diabetes, compared to the broader sample. Thus, this study does not address the prior gaps of biased samples when developing or validating measures, and further acceptability assessment is warranted to explore questionnaire perceptions among diverse subgroups (e.g. those with ethnically diverse background, low English proficiency and/or (health) literacy). Future questionnaire adaptations and design should be informed by, and tested within, the intended population prior to use. Finally, other potentially relevant measures have been excluded from the current study, such as those published in a language other than English and/or novel questionnaires developed and validated since conducting the study. Future research might employ the methods designed here or draw on the identified subthemes to evaluate the acceptability of other measures and / or within populations not incorporated in the current study.
This novel large-scale qualitative study identified user perceptions, and preferred measurement attributes, of five diabetes-specific QoL measures among adults with T1D. Study findings complement our previous psychometric ‘head-to-head’ comparison [ 7 ], and identified the ADDQoL-19, DIDP and Diabetes QOL-Q as more typically incorporating preferred questionnaire attributes. These data, in combination with published development processes and psychometric evaluation, may be used to inform future questionnaire selection as well as best-placed resourcing for the improvement of existing and development novel diabetes-specific QoL measures. For example, findings from the current study directly informed assessment of diabetes-specific QoL within the DAFNE plus (Dose Adjustment For Normal Eating) cluster randomised controlled trial [ 34 ]. Specifically, the ADDQoL was selected as the primary psychosocial outcome measure, reflecting scale acceptability, psychometric performance, as well as existing evidence for scale responsiveness to intervention (including DAFNE [ 35 ]). The DIDP was also included among DAFNE plus assessment tools, with planned assessment of predictive validity and responsiveness to examine appropriateness of this much briefer tool for use in future interventional studies.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Audit of Diabetes-Dependent Quality of Life (ADDQoL-19)
Diabetes Care Profile
DAWN Impact of Diabetes Profile
Diabetes-Specific Quality of Life Scale
Diabetes Quality of Life Questionnaire
- Quality of life
Speight J, Holmes-Truscott E, Hendrieckx C, Skovlund S, Cooke D. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med. 2020;37(3):483–92.
Article CAS PubMed Google Scholar
Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome - A review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26(4):315–27.
Fisher L, Tang T, Polonsky W. Assessing quality of life in diabetes: I. A practical guide to selecting the best instruments and using them wisely. Diabetes Res Clin Pract. 2017;126:278–85.
Article PubMed Google Scholar
Tang TS, Yusuf FL, Polonsky WH, Fisher L. Assessing quality of life in diabetes: II–Deconstructing measures into a simple framework. Diabetes Res Clin Pract. 2017;126:286–302.
Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med. 2002;19(1):1–11.
Skovlund SE, Lichtenberg TH, Hessler D, Ejskjaer N. Can the routine use of patient-reported outcome measures improve the delivery of person-centered Diabetes Care? A review of recent developments and a case study. Curr Diab Rep. 2019;19(9):84.
Holmes-Truscott E, Cooke DD, Hendrieckx C, Coates EJ, Heller SR, Speight J. A comparison of the acceptability and psychometric properties of scales assessing the impact of type 1 diabetes on quality of life—results of ‘YourSAY: quality of life’. Diabet Med. 2021;38(6):e14524.
Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27(5):1159–70.
Article CAS PubMed PubMed Central Google Scholar
The Food and Drug Administration (FDA). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4(79):1–20.
Google Scholar
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14(8):978–88.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Rosal MC, Carbone ET, Goins KV. Use of cognitive interviewing to adapt measurement instruments for low-literate hispanics. Diabetes Educ. 2003;29(6):1006–17.
Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes Metab Res Rev. 2002;18(S3):S64–9.
Hilliard ME, Minard CG, Marrero DG, de Wit M, Thompson D, DuBose SN, et al. Assessing health-related quality of life in children and adolescents with diabetes: development and psychometrics of the type 1 diabetes and life (T1DAL) measures. J Pediatr Psychol. 2020;45(3):328–39.
Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(1–2):79–91.
Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the diabetes care profile. Eval Health Prof. 1996;19(2):208–30.
Peyrot M, Burns KK, Davies M, Forbes A, Hermanns N, Holt R, et al. Diabetes attitudes wishes and needs 2 (DAWN2): a multinational, multi-stakeholder study of psychosocial issues in diabetes and person-centred diabetes care. Diabetes Res Clin Pract. 2013;99(2):174–84.
Holmes-Truscott E, Skovlund SE, Hendrieckx C, Pouwer F, Peyrot M, Speight J. Assessing the perceived impact of diabetes on quality of life: psychometric validation of the DAWN2 impact of Diabetes Profile in the second diabetes MILES-Australia (MILES-2) survey. Diabetes Res Clin Pract. 2019;150:253–63.
Bott U, Mühlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care. 1998;21(5):757–69.
Cooke D, O’Hara MC, Beinart N, Heller S, La Marca R, Byrne M, et al. Linguistic and psychometric validation of the diabetes-specific quality-of-life scale in U.K. English for adults with type 1 diabetes. Diabetes Care. 2013;36(5):1117–25.
Article PubMed PubMed Central Google Scholar
Speight J, Woodcock A, Reaney M, Amiel S, Johnson P, Parrott N, et al. The QoL-Q diabetes’: a novel instrument to assess quality of life for adults with type 1 diabetes undergoing complex interventions including transplantation. Diabet Med. 2010;27:3–4.
Brooks J, McCluskey S, Turley E, King N. The Utility of Template Analysis in qualitative psychology research. Qualitative Res Psychol. 2015;12(2):202–22.
Article Google Scholar
Rattray J, Jones MC. Essential elements of questionnaire design and development. J Clin Nurs. 2007;16(2):234–43.
Speight J, Reaney M, Barnard K. The use of hypothetical scenarios and importance weightings when measuring the impact of diabetes on quality of life. A response to Brose et al. Diabet Med. 2009;26(10):1077–9.
Lenzner T, Kaczmirek L, Lenzner A. Cognitive burden of survey questions and response times: a psycholinguistic experiment. Appl Cogn Psychol. 2010;24(7):1003–20.
Brose LS, Mitchell J, Bradley C. Comments on Speight et al.‘s ‘Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes’. Diabet Med. 2009;26(10):1076–7. author reply 7–9.
Brazier J, Peasgood T, Mukuria C, Marten O, Kreimeier S, Luo N et al. The EQ Health and Wellbeing: overview of the development of a measure of health and wellbeing and key results. Value Health. 2022.
Palamenghi L, Carlucci MM, Graffigna G. Measuring the quality of Life in Diabetic patients: a scoping review. J Diabetes Res. 2020;2020:5419298.
Carr AJ, Higginson IJ. Are quality of life measures patient centred? BMJ. 2001;322(7298):1357–60.
Pickup JC, Harris A. Assessing quality of life for new diabetes treatments and technologies: a simple patient-centered score. J Diabetes Sci Technol. 2007;1(3):394–9.
Meadows K. Interpreting patient-reported outcome measures: narrative and the Fusion of Horizons. Philos Med. 2021;2(2).
Fisher L, Polonsky WH, Perez-Nieves M, Desai U, Strycker L, Hessler D. A new perspective on diabetes distress using the type 2 diabetes distress assessment system (T2-DDAS): prevalence and change over time. J Diabetes Complicat. 2022;36(8):108256.
Article CAS Google Scholar
Polonsky WH, Fisher L, Hessler D, Desai U, King SB, Perez-Nieves M. Toward a more comprehensive understanding of the emotional side of type 2 diabetes: a re-envisioning of the assessment of diabetes distress. J Diabetes Complicat. 2022;36(1):108103.
Coates E, Amiel S, Baird W, Benaissa M, Brennan A, Campbell MJ, Chadwick P, Chater T, Choudhary P, Cooke D, Cooper C. Protocol for a cluster randomised controlled trial of the DAFNEplus (Dose Adjustment For Normal Eating) intervention compared with 5x1 DAFNE: a lifelong approach to promote effective self-management in adults with type 1 diabetes. BMJ open. 2021;11(1):e040438.
Speight J, Amiel SA, Bradley C, Heller S, Oliver L, Roberts S, Rogers H, Taylor C, Thompson G. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment for normal eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled type 1 diabetes. Diabetes Res Clin Pract. 2010;89(1):22–9.
Download references
Acknowledgements
We thank the adults with type 1 diabetes who participated.
This paper summarises independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme, as part of the study ‘Developing and trialling the DAFNE plus (Dose Adjustment for Normal Eating) intervention. A lifelong approach to promote effective self-management in adults with type 1 diabetes’ (Grant Reference Number RP-PG-0514-20013). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. E.H-T, C.H and J.Sp are/were supported by the core funding to the Australian Centre for Behavioural Research in Diabetes provided by the collaboration between Diabetes Victoria and Deakin University.
Author information
Authors and affiliations.
School of Psychology, Deakin University, Geelong, Australia
Elizabeth Holmes-Truscott, Jasmine Schipp, Christel Hendrieckx & Jane Speight
The Australian Centre for Behavioural Research in Diabetes, Diabetes Victoria, Carlton, Australia
Institute for Health Transformation, Deakin University, Geelong, Australia
Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Jasmine Schipp
School of Health Sciences, University of Surrey, Guildford, UK
Debbie D. Cooke
Atlantis Health UK Ltd, London, UK
Sheffield Centre for Health and Related Research (SCHARR), Sheffield CTRU, Sheffield, UK
Elizabeth J. Coates
School of Medicine, Dept of Oncology and Metabolism, University of Sheffield, Sheffield, UK
Simon R. Heller
You can also search for this author in PubMed Google Scholar
Contributions
J.Sp. and D.C. conceived the study and designed it with E.H-T., E.C. and S.H. E.H-T developed the survey and managed data collection. J.Sc. and E.H-T conducted data cleaning and analysis. All authors provided input into interpretation of results. E.H-T. and J.Sc. prepared the first draft of the manuscript. All authors contributed to manuscript revisions and approved the final version.
Corresponding author
Correspondence to Elizabeth Holmes-Truscott .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was received in Australia (Deakin University Human Research Ethics Committee: HEAG-H 03_2017) in accordance with the Australian ‘National Statement on Ethical Conduct in Human Research 2023’. Ethics approval was also received in the UK (Yorkshire & The Humber-Bradford Leeds Research Ethics Committee: 17/YH/0234; Health Research Authority: IRAS ID: 228898). Participants accessed a plain language statement and provided informed consent prior to completing the survey.
Consent for publication
Participants consented to their de-identified data being published for the current purpose.
Competing interests
Jane Speight owns the copyright for ‘Diabetes QOL-Q’. All other authors declare no conflict of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article includes data presented at a scientific meeting: Schipp J, Holmes-Truscott E, Hendrieckx C, Coates E, Heller S, Cooke D, Speight J. A qualitative investigation of the acceptability to adults with type 1 diabetes of five diabetes-specific quality of life measures. Findings of the YourSAY: Quality of Life study. Australasian Diabetes Congress (ADC) 2019, Sydney, Australia, 21-23 August 2019
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Holmes-Truscott, E., Schipp, J., Cooke, D.D. et al. Perceptions of adults with type 1 diabetes toward diabetes-specific quality of life measures: a survey-based qualitative exploration. Health Qual Life Outcomes 22 , 70 (2024). https://doi.org/10.1186/s12955-024-02285-4
Download citation
Received : 19 April 2024
Accepted : 20 August 2024
Published : 02 September 2024
DOI : https://doi.org/10.1186/s12955-024-02285-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Outcome measurement
Health and Quality of Life Outcomes
ISSN: 1477-7525
- Submission enquiries: [email protected]
- Utility Menu
GA4 tracking code

A new therapy for treating Type 1 diabetes
Promising early results show that longstanding harvard stem cell institute (hsci) research may have paved the way for a breakthrough treatment of type 1 diabetes. utilizing research from the melton lab, vertex pharmaceuticals has developed vx-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (t1d). in conjunction with immunosuppressive therapy, vx-880 produced robust restoration of islet cell function on day 90 in the first patient in its phase 1/2 clinical trial..

The patient was treated with a single infusion of VX-880 at half the target dose in conjunction with immunosuppressive therapy. The patient, who was diagnosed with T1D 40 years ago and has been dependent on exogenous (injected) insulin, achieved successful engraftment and demonstrated rapid and robust improvements in multiple measures. These included increases in fasting and stimulated C-peptide, improvements in glycemic control, including HbA1c, and decreases in exogenous insulin requirement, signifying the restoration of insulin-producing islet cells.
VX-880 is not only a potential breakthrough in the treatment of T1D, it is also one of the very first demonstrations of the practical application of embryonic stem cells, using stem cells that have been differentiated into functional islets to treat a patient, explained Doug Melton, Ph.D., co-director of HSCI, is the Xander University Professor at Harvard and an Investigator of the Howard Hughes Medical Institute. Unlike prior treatments, this innovative therapy gives the patient functional hormone producing cells that control glucose metabolism. This potentially obviates the lifelong need for patients with diabetes to self-inject insulin as the replacement cells “provide the patient with the natural factory to make their own insulin,” explained Melton.
These results from the first patient treated with VX-880 are unprecedented. What makes these results truly remarkable is that they were achieved with only half the target dose,” said Bastiano Sanna, Ph.D., Executive Vice President and Chief of Cell and Genetic Therapies at Vertex. “While still early, these results support the continued progression of our VX-880 clinical studies, as well as future studies using our encapsulated islet cells, which hold the potential to be used without the need for immunosuppression.”
“As a surgeon who has worked in the field of islet cell transplantation for decades, this approach, which obviates the need for an organ donor, could be a game changer,” said James Markmann, M.D., Ph.D., Professor of Surgery and Chief of the Division of Transplant Surgery at Massachusetts General Hospital. “We are excited to progress this unique and potentially transformative medicine through clinical trials and to patients.”
“More than a decade ago our lab had a vision for developing an islet cell replacement therapy to provide a functional cure to people suffering from T1D,” said Melton, a founder and one of the first co-chairs of the Harvard Stem Cell and Regenerative Biology Department. “These promising results bring great hope that stem cell-derived, fully differentiated islet cells could deliver a life-changing therapy for people who suffer from the relentless life-long burden of T1D. I'm so grateful that Harvard and the Harvard Stem Cell Institute have supported this work.”
Latest News
How a small fish could lead to better strategies to repair tendon tears, jason buenrostro lands macarthur ‘genius grant’, new model for in vitro production of human brown fat cells lays groundwork for obesity, diabetes cell therapy, share this story, news by topic.
- Alzheimer's Disease (8)
- Bioengineering (31)
- Blood Diseases (52)
- Cancer (55)
- Cardiovascular Disease (25)
- Diabetes (30)
- Endocrine Disease (1)
- Eye Diseases (10)
- Fibrosis (6)
- Gastrointestinal Disease (8)
- Genetics/Epigenetics (24)
- Graft-Versus-Host Disease (1)
- Hearing Loss (5)
- Immunology (15)
- Kidney Disease (12)
- Liver Disease (6)
- Lung Disease (9)
- Multiple Sclerosis (1)

Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies
- Download PDF Copy

Unlocking the potential of renewable β cells and precision gene editing, researchers aim to revolutionize diabetes care and bring us closer to a functional cure for type 1 diabetes.

In a recent study published in the journal Nature Reviews Endocrinology , researchers examined the progress in cell replacement therapies for type 1 diabetes mellitus (T1DM), focusing on generating replenishable β cells, improving transplantation methods, and addressing challenges related to immune modulation and clinical application.
T1DM affects 8.75 million people globally, approximately 1.52 million patients under 20. T1DM results from the autoimmune destruction of pancreatic β cells, consequent insulin insufficiency, and chronic hyperglycemia. Although glucose monitoring and insulin dosing help manage the disease, achieving optimal glycemic control remains challenging. Pancreatic islet or β cell transplantation offers a potential cure but faces challenges such as limited donor availability, poor cell engraftment, and the need for lifelong immunosuppression. Current research focuses on improving cell delivery, finding alternative cell sources, and reducing reliance on immunosuppression. In the present review, researchers discussed the current advancements in cell transplantation for T1DM, focusing on β cell generation, delivery technologies, immune modulation, relevant animal models, and the clinical translation of these therapies.
Renewable islet cell sources
The limited availability of donor islets has driven the development of stem cell-derived islets as a renewable source for T1DM therapy. These islets, generated from human pluripotent stem cells (hPSCs), show promise in clinical trials but remain challenged by functional immaturity, transcriptional identity issues, and the inability to control the ratio of β, α, and δ cells. During in vitro differentiation, a significant number of cells may acquire an unwanted identity, resembling serotonin-producing enterochromaffin cells, which complicates their application in T1DM therapy. While in vivo transplantation can enhance the function of these cells, optimizing in vitro production processes and ensuring safety, particularly concerning uncommitted cell types that could form tumors and ensuring genetic stability, remains crucial. Advances in scalable manufacturing, characterization protocols, and cryopreservation will be essential for the clinical adoption and accessibility of these therapies.
Cell delivery strategies
Pancreatic islet transplantation for T1DM involves strategies like microencapsulation and macroencapsulation to protect islet cells and enhance their function. Microencapsulation encloses cells in gel-like microspheres, allowing nutrient exchange while shielding them from immune attacks. However, challenges like inflammation and fibrotic overgrowth affect long-term viability. Macroencapsulation delivers larger cell doses in retrievable units but faces issues with oxygen supply and fibrosis. Open devices and scaffolds aim for direct vascularization of grafts to improve integration and function, using approaches like simultaneous implant-transplant methods, decellularized tissue scaffolds, and 3D-printed architectures. Prevascularization systems are also explored to establish a vascular network before cell transplantation, improving cell survival and reducing immune responses. Despite these innovations, ensuring adequate mass transfer of oxygen, glucose, and insulin within encapsulation devices and managing graft size and immune protection remain significant hurdles. While these approaches show promise, challenges remain in achieving long-term efficacy , minimizing immune rejection, and optimizing oxygen and nutrient delivery to transplanted cells.
Alternative immunoprotection methods
β cell replacement faces challenges distinct from non-autoimmune diseases, mainly due to the need to prevent autoimmunity recurrence. Current immunosuppressive therapies are effective but have severe side effects, including organ toxicity and increased infection risks. Emerging strategies focus on more targeted, less toxic immunomodulation. These include biomaterial-based localized drug delivery, islet co-delivery with immunomodulatory cells, and reducing islet graft immunogenicity through advanced gene editing techniques. Biomaterials can deliver immunomodulatory drugs directly to the transplant site, while co-delivery with cells like mesenchymal stem cells improves islet survival. Gene editing technologies, such as CRISPR–Cas9, are being utilized to engineer hypoimmune islet grafts by knocking down immunogenic markers or overexpressing protective signals. However, the long-term impact of these genetic modifications remains uncertain, and safety concerns persist regarding the potential for immune evasion by these modified cells.
Animal models
Animal models support the development of cell transplantation strategies and immunomodulatory interventions. Immunocompromised models, mainly using mice, allow for studying human islet engraftment without rejection. In contrast, immunocompetent models, such as rats, pigs, and non-human primates (NHPs), better mimic human immune responses critical for evaluating inflammatory and immune protection strategies. Humanized models, incorporating human immune components, provide a unique platform to assess the immunogenicity of β cell grafts and therapeutic interventions despite limitations such as graft-versus-host disease and shorter experimental timeframes. Pigs provide insights into islet transplantation due to their physiological similarities to humans, while NHPs serve as valuable translational models, contributing to understanding immune responses and developing new immunosuppressive strategies. Together, these models facilitate comprehensive assessments of therapeutic interventions for T1DM.
Clinical translation
Harmonizing preclinical testing protocols is vital for β cell replacement therapy development. Characterization of stem cell-derived β cells should include composition and functional assessments. Initial rodent studies must evaluate cell delivery and immune responses, with further validation in larger animal models. Development of a β cell replacement product involves renewable cell sources, effective delivery, and immune rejection prevention. The goal is to create a safe, reproducible product that restores glycemic control for over ten years without systemic immunosuppression.
Conclusion and outlook
In conclusion, cell transplantation for T1DM has evolved significantly, with stem cell-derived islets showing promise for clinical application. However, challenges related to cell composition, functional maturity, and long-term safety continue to be critical areas of focus. Collaborative consortia are accelerating progress by integrating complementary technologies. Innovations in renewable β cell sources, gene editing technology, and subcutaneous transplantation methods aim to improve cell delivery, immune modulation, and patient outcomes. The goal is to develop a widely applicable, practical, and accessible treatment that improves the quality of life for T1DM patients.
- Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead. Grattoni, A. et al., Nature Reviews Endocrinology (2024), DOI: 10.1038/s41574-024-01029-0, https://www.nature.com/articles/s41574-024-01029-0
Posted in: Medical Science News | Medical Research News | Medical Condition News
Tags: Autoimmunity , Cas9 , Cell , Chronic , CRISPR , Diabetes , Diabetes Mellitus , Drug Delivery , Drugs , Efficacy , Endocrinology , Fibrosis , Gene , Genetic , Glucose , Hyperglycemia , Immunomodulatory , Immunosuppression , in vitro , in vivo , Inflammation , Insulin , Manufacturing , Mesenchymal Stem Cells , Oxygen , Preclinical , Preclinical Testing , Research , Serotonin , Stem Cells , Technology , Translation , Transplant , Type 1 Diabetes , Vascular

Dr. Sushama R. Chaphalkar
Dr. Sushama R. Chaphalkar is a senior researcher and academician based in Pune, India. She holds a PhD in Microbiology and comes with vast experience in research and education in Biotechnology. In her illustrious career spanning three decades and a half, she held prominent leadership positions in academia and industry. As the Founder-Director of a renowned Biotechnology institute, she worked extensively on high-end research projects of industrial significance, fostering a stronger bond between industry and academia.
Please use one of the following formats to cite this article in your essay, paper or report:
Chaphalkar, Sushama R.. (2024, September 05). Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies. News-Medical. Retrieved on September 06, 2024 from https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx.
Chaphalkar, Sushama R.. "Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies". News-Medical . 06 September 2024. <https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx>.
Chaphalkar, Sushama R.. "Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies". News-Medical. https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx. (accessed September 06, 2024).
Chaphalkar, Sushama R.. 2024. Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies . News-Medical, viewed 06 September 2024, https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx.
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

Cure-Focused Diabetes Research
The Diabetes Research Institute houses teams of scientists, engineers, and clinicians with the expertise required to tackle diabetes from many angles. This integration of medicine and technology drives the vision behind the DRI strategy, a comprehensive, multidisciplinary approach to cure diabetes. The strategy builds upon decades of cure-focused research and addresses the major challenges that stand in the way of a biological cure.
A cure is within reach.
A cure would mean restoring natural insulin production and normalizing blood sugar levels without imposing other risks.
DRI clinical trials are already dramatically improving the lives of some people with type 1 diabetes who are now living insulin-free.
The Institute’s scientists are addressing the major research challenges that stand in the way of a biological cure. But continuing this research is only possible with your support.
News & Events
Visit the DRI Foundation Virtual Tour >
Tour the DRI’s facilities and meet our team in the metaverse! Learn how you can help us find a cure…

Inside Our Labs…

Hamptons Garden Gala…

Diabetes Research…
Get the latest news in your inbox.
Hear from our team.
Meet our team in the search for a cure. Hear the stories of the researchers, patients, and supporters who are helping drive our research forward.

Diabetes and Family…

Advancements and…

DRIF 50 Years…
Help our team find a cure.
Dri virtual tour.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Year in Review
- Published: 06 December 2023
Diabetes mellitus in 2023
Type 1 diabetes mellitus: a brave new world
- Pieter-Jan Martens 1 &
- Chantal Mathieu ORCID: orcid.org/0000-0002-4055-5233 1
Nature Reviews Endocrinology volume 20 , pages 71–72 ( 2024 ) Cite this article
1420 Accesses
2 Citations
5 Altmetric
Metrics details
- Pre-diabetes
- Type 1 diabetes
One hundred years after the Nobel prize was bestowed on Banting and McLeod for the ‘discovery’ of insulin, we are again seeing major evolutions in the management of type 1 diabetes mellitus, with the prospect of achieving disease control beyond mere management now becoming real. Here, we discuss the latest, most notable developments.
Key advances
Automated insulin delivery improves glycaemic control and pregnancy outcomes in pregnancy complicated by maternal type 1 diabetes mellitus (T1DM) compared to standard of care 2 .
Once-weekly basal insulin is non-inferior compared to its once-daily counterpart, in terms of HbA 1c (ref. 5 ).
Whole-blood gene expression signatures can help in stratifying the heterogeneity of T1DM 6 .
In patients with T1DM, detection of disease earlier in its course rather than later yields a more favourable clinical outcome at symptom onset 8 .
Teplizumab not only preserves β-cell function at stage 2 T1DM 7 , but also at stage 3 (symptom-onset) T1DM 10 .
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
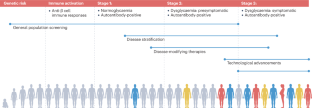
Phillip, M. et al. Consensus recommendations for the use of automated insulin delivery technologies in clinical practice. Endocr. Rev. 44 , 254–280 (2023).
Article PubMed Google Scholar
Lee, T. T. M. et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N. Engl. J. Med. 389 , 1566–1578 (2023).
Article CAS PubMed Google Scholar
Ekberg, N. R., Hartvig, N. V., Kaas, A., Møller, J. B. & Adolfsson, P. Smart pen exposes missed basal insulin injections and reveals the impact on glycemic control in adults with type 1 diabetes. J. Diabetes Sci. Technol. https://doi.org/10.1177/19322968221104142 (2022).
Kazda, C. M. et al. Novel once-weekly basal insulin Fc achieved similar glycemic control with a safety profile comparable to insulin degludec in patients with type 1 diabetes. Diabetes Care 46 , 1052–1059 (2023).
Article CAS PubMed PubMed Central Google Scholar
Russell-Jones, D. et al. Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial. Lancet 402 , 1636–1647 (2023).
Suomi, T. et al. Gene expression signature predicts rate of type 1 diabetes progression. EBioMedicine 92 , 104625 (2023).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381 , 603–613 (2019).
Hummel, S. et al. Children diagnosed with presymptomatic type 1 diabetes through public health screening have milder diabetes at clinical manifestation. Diabetologia 66 , 1633–1642 (2023).
Sims, E. K. et al. Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes 71 , 610–623 (2022).
Ramos, E. L. et al. Teplizumab and β-cell function in newly diagnosed type 1 diabetes. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2308743 (2023).
Download references
Author information
Authors and affiliations.
Endocrinology Department, UZ Leuven, Leuven, Belgium
Pieter-Jan Martens & Chantal Mathieu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Chantal Mathieu .
Ethics declarations
Competing interests.
C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres, Mannkind, Sandoz and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Imcyse, Novo Nordisk, Sanofi and ActoBio Therapeutics; C.M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Astra Zeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. C.M. is president of EASD. All external support of EASD is to be found on http://www.easd.org/ . P.M. has no competing interests.
Additional information
Related links.
INNODIA: www.innodia.eu
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Martens, PJ., Mathieu, C. Type 1 diabetes mellitus: a brave new world. Nat Rev Endocrinol 20 , 71–72 (2024). https://doi.org/10.1038/s41574-023-00936-y
Download citation
Published : 06 December 2023
Issue Date : February 2024
DOI : https://doi.org/10.1038/s41574-023-00936-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Patient Care & Health Information
- Diseases & Conditions
- Type 1 diabetes
What is type 1 diabetes? A Mayo Clinic expert explains
Learn more about type 1 diabetes from endocrinologist Yogish Kudva, M.B.B.S.
I'm Dr. Yogish C. Kudva an endocrinologist at Mayo Clinic. In this video, we'll cover the basics of type 1 diabetes. What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love. We are here to give you the best information available. Type 1 diabetes is a chronic condition that affects the insulin making cells of the pancreas. It's estimated that about 1.25 million Americans live with it. People with type 1 diabetes don't make enough insulin. An important hormone produced by the pancreas. Insulin allows your cells to store sugar or glucose and fat and produce energy. Unfortunately, there is no known cure. But treatment can prevent complications and also improve everyday life for patients with type 1 diabetes. Lots of people with type 1 diabetes live a full life. And the more we learn and develop treatment for the disorder, the better the outcome.
We don't know what exactly causes type 1 diabetes. We believe that it is an auto-immune disorder where the body mistakenly destroys insulin producing cells in the pancreas. Typically, the pancreas secretes insulin into the bloodstream. The insulin circulates, letting sugar enter your cells. This sugar or glucose, is the main source of energy for cells in the brain, muscle cells, and other tissues. However, once most insulin producing cells are destroyed, the pancreas can't produce enough insulin, meaning the glucose can't enter the cells, resulting in an excess of blood sugar floating in the bloodstream. This can cause life-threatening complications. And this condition is called diabetic ketoacidosis. Although we don't know what causes it, we do know certain factors can contribute to the onset of type 1 diabetes. Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly increased risk of developing it. Genetics. The presence of certain genes can also indicate an increased risk. Geography. Type 1 diabetes becomes more common as you travel away from the equator. Age, although it can occur at any age there are two noticeable peaks. The first occurs in children between four and seven years of age and the second is between 10 and 14 years old.
Signs and symptoms of type 1 diabetes can appear rather suddenly, especially in children. They may include increased thirst, frequent urination, bed wetting in children who previously didn't wet the bed. Extreme hunger, unintended weight loss, fatigue and weakness, blurred vision, irritability, and other mood changes. If you or your child are experiencing any of these symptoms, you should talk to your doctor.
The best way to determine if you have type 1 diabetes is a blood test. There are different methods such as an A1C test, a random blood sugar test, or a fasting blood sugar test. They are all effective and your doctor can help determine what's appropriate for you. If you are diagnosed with diabetes, your doctor may order additional tests to check for antibodies that are common in type 1 diabetes in the test called C-peptide, which measures the amount of insulin produced when checked simultaneously with a fasting glucose. These tests can help distinguish between type 1 and type 2 diabetes when a diagnosis is uncertain.
If you have been diagnosed with type 1 diabetes, you may be wondering what treatment looks like. It could mean taking insulin, counting carbohydrates, fat protein, and monitoring your glucose frequently, eating healthy foods, and exercising regularly to maintain a healthy weight. Generally, those with type 1 diabetes will need lifelong insulin therapy. There are many different types of insulin and more are being developed that are more efficient. And what you may take may change. Again, your doctor will help you navigate what's right for you. A significant advance in treatment from the last several years has been the development and availability of continuous glucose monitoring and insulin pumps that automatically adjust insulin working with the continuous glucose monitor. This type of treatment is the best treatment at this time for type 1 diabetes. This is an exciting time for patients and for physicians that are keen to develop, prescribe such therapies. Surgery is another option. A successful pancreas transplant can erase the need for additional insulin. However, transplants aren't always available, not successful and the procedure can pose serious risks. Sometimes it may outweigh the dangers of diabetes itself. So transplants are often reserved for those with very difficult to manage conditions. A successful transplant can bring life transforming results. However, surgery is always a serious endeavor and requires ample research and concentration from you, your family, and your medical team.
The fact that we don't know what causes type 1 diabetes can be alarming. The fact that we don't have a cure for it even more so. But with the right doctor, medical team and treatment, type 1 diabetes can be managed. So those who live with it can get on living. If you would like to learn even more about type 1 diabetes, watch our other related videos or visit mayoclinic.org. We wish you well.
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. In this condition, the pancreas makes little or no insulin. Insulin is a hormone the body uses to allow sugar (glucose) to enter cells to produce energy.
Different factors, such as genetics and some viruses, may cause type 1 diabetes. Although type 1 diabetes usually appears during childhood or adolescence, it can develop in adults.
Even after a lot of research, type 1 diabetes has no cure. Treatment is directed toward managing the amount of sugar in the blood using insulin, diet and lifestyle to prevent complications.
Products & Services
- A Book: The Essential Diabetes Book
Type 1 diabetes symptoms can appear suddenly and may include:
- Feeling more thirsty than usual
- Urinating a lot
- Bed-wetting in children who have never wet the bed during the night
- Feeling very hungry
- Losing weight without trying
- Feeling irritable or having other mood changes
- Feeling tired and weak
- Having blurry vision
When to see a doctor
Talk to your health care provider if you notice any of the above symptoms in you or your child.
The exact cause of type 1 diabetes is unknown. Usually, the body's own immune system — which normally fights harmful bacteria and viruses — destroys the insulin-producing (islet) cells in the pancreas. Other possible causes include:
- Exposure to viruses and other environmental factors
The role of insulin
Once a large number of islet cells are destroyed, the body will produce little or no insulin. Insulin is a hormone that comes from a gland behind and below the stomach (pancreas).
- The pancreas puts insulin into the bloodstream.
- Insulin travels through the body, allowing sugar to enter the cells.
- Insulin lowers the amount of sugar in the bloodstream.
- As the blood sugar level drops, the pancreas puts less insulin into the bloodstream.
The role of glucose
Glucose — a sugar — is a main source of energy for the cells that make up muscles and other tissues.
- Glucose comes from two major sources: food and the liver.
- Sugar is absorbed into the bloodstream, where it enters cells with the help of insulin.
- The liver stores glucose in the form of glycogen.
- When glucose levels are low, such as when you haven't eaten in a while, the liver breaks down the stored glycogen into glucose. This keeps glucose levels within a typical range.
In type 1 diabetes, there's no insulin to let glucose into the cells. Because of this, sugar builds up in the bloodstream. This can cause life-threatening complications.
Risk factors
Some factors that can raise your risk for type 1 diabetes include:
- Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly higher risk of developing the condition.
- Genetics. Having certain genes increases the risk of developing type 1 diabetes.
- Geography. The number of people who have type 1 diabetes tends to be higher as you travel away from the equator.
- Age. Type 1 diabetes can appear at any age, but it appears at two noticeable peaks. The first peak occurs in children between 4 and 7 years old. The second is in children between 10 and 14 years old.
Complications
Over time, type 1 diabetes complications can affect major organs in the body. These organs include the heart, blood vessels, nerves, eyes and kidneys. Having a normal blood sugar level can lower the risk of many complications.
Diabetes complications can lead to disabilities or even threaten your life.
- Heart and blood vessel disease. Diabetes increases the risk of some problems with the heart and blood vessels. These include coronary artery disease with chest pain (angina), heart attack, stroke, narrowing of the arteries (atherosclerosis) and high blood pressure.
Nerve damage (neuropathy). Too much sugar in the blood can injure the walls of the tiny blood vessels (capillaries) that feed the nerves. This is especially true in the legs. This can cause tingling, numbness, burning or pain. This usually begins at the tips of the toes or fingers and spreads upward. Poorly controlled blood sugar could cause you to lose all sense of feeling in the affected limbs over time.
Damage to the nerves that affect the digestive system can cause problems with nausea, vomiting, diarrhea or constipation. For men, erectile dysfunction may be an issue.
- Kidney damage (nephropathy). The kidneys have millions of tiny blood vessels that keep waste from entering the blood. Diabetes can damage this system. Severe damage can lead to kidney failure or end-stage kidney disease that can't be reversed. End-stage kidney disease needs to be treated with mechanical filtering of the kidneys (dialysis) or a kidney transplant.
- Eye damage. Diabetes can damage the blood vessels in the retina (part of the eye that senses light) (diabetic retinopathy). This could cause blindness. Diabetes also increases the risk of other serious vision conditions, such as cataracts and glaucoma.
- Foot damage. Nerve damage in the feet or poor blood flow to the feet increases the risk of some foot complications. Left untreated, cuts and blisters can become serious infections. These infections may need to be treated with toe, foot or leg removal (amputation).
- Skin and mouth conditions. Diabetes may leave you more prone to infections of the skin and mouth. These include bacterial and fungal infections. Gum disease and dry mouth also are more likely.
- Pregnancy complications. High blood sugar levels can be dangerous for both the parent and the baby. The risk of miscarriage, stillbirth and birth defects increases when diabetes isn't well-controlled. For the parent, diabetes increases the risk of diabetic ketoacidosis, diabetic eye problems (retinopathy), pregnancy-induced high blood pressure and preeclampsia.
There's no known way to prevent type 1 diabetes. But researchers are working on preventing the disease or further damage of the islet cells in people who are newly diagnosed.
Ask your provider if you might be eligible for one of these clinical trials. It is important to carefully weigh the risks and benefits of any treatment available in a trial.
- Summary of revisions: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-Srev.
- Papadakis MA, et al., eds. Diabetes mellitus. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed May 4, 2022.
- What is diabetes? National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed May 4, 2022.
- Levitsky LL, et al. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes mellitus (DM). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed May 4, 2022.
- AskMayoExpert. Type 1 diabetes mellitus. Mayo Clinic; 2021.
- Robertson RP. Pancreas and islet transplantation in diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Levitsky LL, et al. Management of type 1 diabetes mellitus in children during illness, procedures, school, or travel. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Hyperglycemia (high blood glucose). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hyperglycemia. Accessed May 4, 2022.
- Diabetes and DKA (ketoacidosis). American Diabetes Association. https://www.diabetes.org/diabetes/dka-ketoacidosis-ketones. Accessed May 4, 2022.
- Insulin resistance & prediabetes. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance. Accessed May 4, 2022.
- Blood sugar and insulin at work. American Diabetes Association. https://www.diabetes.org/tools-support/diabetes-prevention/high-blood-sugar. Accessed May 4, 2022.
- Inzucchi SE, et al. Glycemic control and vascular complications in type 1 diabetes. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes and oral health. American Diabetes Association. https://www.diabetes.org/diabetes/keeping-your-mouth-healthy. Accessed May 4, 2022.
- Drug treatment of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/drug-treatment-of-diabetes-mellitus. Accessed May 4, 2022.
- Weinstock DK, et al. Management of blood glucose in adults with type 1 diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 7, 2022.
- FDA proves first automated insulin delivery device for type 1 diabetes. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulin-delivery-device-type-1-diabetes. Accessed May 4, 2022.
- Boughton CK, et al. Advances in artificial pancreas systems. Science Translational Medicine. 2019; doi:10.1126/scitranslmed.aaw4949.
- Hypoglycemia (low blood sugar). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hypoglycemia. Accessed May 4, 2022.
- Diabetes in the workplace and the ADA. U.S. Equal Opportunity Employment Commission. https://www.eeoc.gov/laws/guidance/diabetes-workplace-and-ada. Accessed May 4, 2022.
- Cardiovascular disease and risk management: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S010.
- Diabetes technology. Standards of Medical Care in Diabetes — 2022. 2022; doi:10.2337/dc22-S007.
- FDA authorizes a second artificial pancreas system. JDRF. https://www.jdrf.org/blog/2019/12/13/jdrf-reports-fda-authorizes-second-artificial-pancreas-system/. Accessed May 4, 2022.
- Classification and diagnosis of diabetes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S002.
- Retinopathy, neuropathy, and foot care: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Glycemic targets: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S009.
- Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S005.
- Centers for Disease Control and Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2011;60:1709.
- Management of diabetes in pregnancy: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S015.
- Older adults: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S013.
- FDA approves first-of-its-kind automated insulin delivery and monitoring system for use in young pediatric patients. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric#:~:text=Today, the U.S. Food and,by individuals aged 2 to. Accessed May 8, 2022.
- What you need to know: Getting a COVID-19 vaccine. American Diabetes Association. https://www.diabetes.org/coronavirus-covid-19/vaccination-guide. Accessed June 1, 2022.
News from Mayo Clinic
- Driven by family, fueled by hope: Mayo Clinic researcher fights against Type 1 diabetes Aug. 06, 2023, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Talk to us about diabetes
0345 123 2399
customer support
Your top priorities for research into type 1 diabetes revealed

Hundreds of people with type 1 diabetes, their families and healthcare professionals have chosen their most pressing research priorities for type 1 diabetes. The top ten priorities will help to guide future type 1 diabetes research in the UK and Ireland to make sure it has the greatest possible benefit for people with the condition.
As the UK’s largest charitable funder of diabetes research, it’s critical that our research funds address the specific challenges and needs of people with diabetes and those who care for them.
It’s also critical that we make sure others - academics, healthcare professionals and other research funders – hear these views loud and clear and act upon them.
We work with the Priority Setting Partnership (PSP) initiative, run by the James Lind Alliance (JLA) and supported by the National Institute for Health Research (NIHR) , to help bring the views of people with real-life experience of diabetes into research.
Through surveys and workshops, this initiative finds and prioritises their most pressing concerns and questions that can be answered through research. Diabetes UK contributed to the first Type 1 Diabetes PSP in 2011, the Diabetes and Pregnancy PSP in 2020 , and led the Type 2 Diabetes PSP in 2017.
Your new top ten priorities
Since the last Type 1 Diabetes PSP in 2011 there's been some big changes in type 1 treatment and care , so the priorities were due an update. The latest Type 1 PSP – which condensed and whittled down nearly 3000 questions submitted by people affected by type 1 to a shortlist of the top ten – has just been published . And here they are:
1. Can the use of artificial intelligence or faster acting insulins help achieve fully closed loop insulin delivery?
2. Is time in range a better predictor of diabetes management and complications compared to HbA1c (an average reading of blood sugar over a 3-month period)?
3. What impact do hormonal phases such as the perimenstrual period and menopause play in glycaemic management and what treatments are most effective for managing glucose levels around these times?
4. What interventions are the most effective for reducing diabetes related distress and burnout?
5. What are the long-term implications of frequent hypoglycaemia on physical and mental health?
6. What impact does type 1 diabetes (including frequent low blood sugar) have on memory and cognition in older adults?
7. How can health care professionals better take into account the physical, psychological and social aspects of type 1 diabetes in clinics?
8. How can access to potential therapies like stem cell therapy, transplants and medications that modify the immune systems be improved so that everyone with type 1 diabetes can be guaranteed access?
9. Why do some people with type 1 diabetes become insulin resistant and does resistance increase with the number of years a person has diabetes and if so, why?
10. Can technology assist to accurately count carbohydrates without having to weigh or measure all foods and drink?
Dr Christine Newman, Lead Clinical Researcher at the Health Research Board Diabetes Collaborative Clinical Trial Network in Ireland who funded the PSP, emphasised the importance of these findings:
“This study is a powerful example of how Public and Patient Involvement can shape the future of healthcare. This work highlights the real-world challenges and unmet needs of adults living with Type 1 diabetes. By focusing on these top ten priorities, we can ensure that future research and healthcare services are aligned with what truly matters to those affected by the condition.”
We will use these top 10 research priorities in the decisions it makes about how research is funded, and they will inform the work of the Diabetes Research Steering Groups .
We will also publicise these priorities widely to researchers and organisations that fund diabetes research. The priorities could influence those who work in universities and academic institutions, government agencies or in industry.
Dr Elizabeth Robertson, Director of Research at Diabetes UK, explains:
“We need to make sure research that we fund has the greatest possible benefit for people with diabetes. Knowing the most important priorities of people living with or treating type 1 diabetes will help us direct funding to where it’s needed most.”
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

Best exercises for people with type 1 diabetes outlined in new research

Daily Pastry Consumption Could Silently Harm the Heart Within a Month
Conor seery, share article.
Researchers have revealed what types of exercise suit people living with type 1 diabetes .
Academics from the Universidade Federal do Vale do São Francisco in partnership with Staffordshire University have outlined that gender determines what exercise an individual with type 1 diabetes should do.
Senior author Dr Pooya Soltani said: “This study is important because people with diabetes often lack motivation to exercise as a means of managing their condition.
“One reason for this is that physical activity can lead to blood sugar drops, causing discomfort and demotivation. We investigated whether the type of physical activity could mitigate these blood sugar drops .”
- Children whose mothers have type 1 diabetes less likely to have condition than if their father does
- Type 1 diabetes among youths with presymptomatic symptoms was higher during pandemic
- Type 1 diabetes risk after age 10 differs between boys and girls
A total of 19 adults with type 1 diabetes took part in the study. The research team looked at each participant’s glycaemic and cardiovascular responses following interval exercise sessions and continuous exercise sessions.
Each person involved in the trial participated in half an hour of moderate aerobic exercise on a treadmill.
Meanwhile, the interval aerobic exercise session included alternating one-minute intervals at 40% and 60% of estimated maximal oxygen consumption (VO 2max ). Whereas the participants performed the continuous exercise session at 50% of VO 2max .
The researchers analysed the participant’s blood pressure, heart rate and blood glucose levels prior to and instantly after the exercise sessions, as well as 20 minutes after.
According to the study, greater blood glucose reductions were seen in men than women after continuous aerobic exercise and interval exercise.
Women only reduced their blood glucose values after continuous exercise, and not interval exercise, the results have reported.
Fellow author Dr Jorge Luiz de Brito-Gomes said: “Our study shows that for male patients, interval exercise, such as short bursts of walking, is preferable when starting with low blood sugar levels.
- Time in Range
- Guide to HbA1c
- Blood Sugar Level Ranges
“Conversely, continuous exercise, like running, is more suitable for those with higher initial blood sugar levels. These approaches can help prevent sudden blood sugar drops.”
Dr Luiz de Brito-Gomes added: “For female patients, both interval and continuous aerobic exercise appear to be effective starting points.
“We hope these findings show that gender-specific recommendations should be considered for aerobic exercise prescription, especially for men with irregular physical activity levels.”
Read the study here .
What's new on the forum? ⭐️
What have you eaten today (low carb forum) low-carb diet forum.
Administrator
Prediabetes questions Prediabetes
Locum Trouble Type 1 Diabetes
Fasting hypoglycemia Reactive Hypoglycemia
Catkysydney
OVER 6 YEARS IN OFFICIAL REMISSION THANKS TO THIS FORUM - SHARING WHAT WORKS FOR ME Success Stories and Testimonials
Dexcom one+ Apple Watch question Blood Glucose Monitoring
Reading vary every morning Ask A Question
A1c question Newly Diagnosed
Freestyle 2 compatible with update of 17.6.1 Ask A Question
Hi I have a question about Dr Greger author of, How not to die. And exponent of the plant-based diet, he says fat causes diabetes not carbs. Newly Diagnosed
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.
You May Also Like

- Uncategorised
Public Health England considers low carb approach for type 2 diabetes
- 2 minute read

- Coronavirus
Conversation about doctors’ appointments occurring virtually rumbles on
- 3 minute read

Coronavirus: UK instructed to stay at home this weekend
- 1 minute read
- Collections
- Diabetes Vic Clinic
- Book a Program
- phone-solid
- Member Login
- Donate Donate
- Languages Shape

1300 437 386
NDSS Helpline
1800 637 700
Member Hub Login
Please login to enjoy your exclusive member hub!
Username or Email Address
Forgot your password?
Not a member? JOIN NOW to access exclusive member benefits!
Having trouble? Visit our Online Help section
Find out other ways to get involved
World-first trial offers new hope for type 1 diabetes
On this page, more blogs dropdown.
- Diabetes management
- Physical activity

Research breakthrough
Researchers at St Vincent’s Institute of Medical Research (SVI) have shown that a commonly used rheumatoid arthritis drug can slow down the progression of type 1 diabetes.
The world-first human trial, led by SVI’s Professors Thomas Kay and Helen Thomas, showed that a drug called baricitinib can safely preserve the body’s own insulin production and slow down the progression of type 1 diabetes in people who start the treatment within 100 days of diagnosis.
Introducing Professor Kay
“When type 1 diabetes is first diagnosed there is a large number of insulin-producing cells still present,” Professor Kay explains.
“We wanted to see if we could protect further destruction of these cells by the immune system. We showed that baricitinib is safe and effective at slowing the progression of type 1 diabetes in people who have been recently diagnosed.”
This groundbreaking research shows promise as the first disease-modifying treatment of its kind for type 1 diabetes that can be delivered as a tablet.
“It is tremendously exciting for us to be the first group anywhere in the world to test the efficacy of baricitinib as a potential type 1 diabetes treatment,” Professor Kay says.
“Up until now, people with type 1 diabetes have been reliant on insulin delivered via injection or infusion pump.
Our trial showed that, if started early enough after diagnosis, and while the participants remained on the medication, their production of insulin was maintained. People with type 1 diabetes in the trial who were given the drug required significantly less insulin for treatment.”
Management of the lifelong autoimmune disease is incredibly burdensome and requires careful glucose monitoring and insulin injections (or use of a pump) day and night to stay alive.
Until insulin’s discovery more than 100 years ago, type 1 diabetes was a fatal condition. While insulin is lifesaving, it can be dangerous if too much or too little is delivered. Diabetes also comes with a higher risk of long-term complications, including heart attack and stroke, vision impairment, kidney disease and nerve damage.
“We are very optimistic that this treatment will become clinically available. This would be a huge step-change in how type 1 diabetes is managed and we believe it shows promise as a fundamental improvement in the ability to control type 1 diabetes,” says Professor Helen Thomas, preclinical lead on the trial.
The clinical trial was funded by Juvenile Diabetes Research Foundation (JDRF), the leading type 1 diabetes research, advocacy, and community programs organisation. Partners included The Royal Melbourne Hospital, St Vincent’s Hospital Melbourne, The Royal Children’s Hospital and The Women’s and Children’s Hospital in Adelaide.
For more information about participating in diabetes research, please click here .
To donate to diabetes research, visit our Donate page . Article published February 2024.
Latest Blogs Articles

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- For Health Professionals
- Diabetes Discoveries & Practice Blog
How Is Diabetes Different for Older Adults?
- Type 1 Diabetes
- Complications of Diabetes

Learn about some of the unique challenges for older adults with diabetes and how health care professionals can help them manage their care.
Experts expect the number of older adults—people ages 65 and older—in the United States living with type 1 or type 2 diabetes to increase rapidly in the coming decades. Dennis T. Villareal, MD, a physician-scientist with specialty training in geriatrics and endocrinology, discusses the medical, psychological, functional, and social needs of older adults with diabetes, and the role of emerging technologies.
Q: What is the current prevalence of diabetes—type 1 and type 2—in older adults in the United States?
A: More than a quarter of people over age 65 have diabetes. Although type 2 diabetes is the most common type of diabetes in older adults, type 1 diabetes is becoming more common as people with the disease are living longer because of improved insulin delivery, technology, and care. We expect the number of older adults living with type 1 or type 2 diabetes to increase markedly in the coming decades.
Q: What are some issues to consider when managing diabetes in older adults? Why is it important to tailor how health care professionals manage diabetes in older adults?
A: Health care professionals and health care teams not only need to regularly assess medical care, but also psychological function and social challenges to ensure quality of life. For example, it’s imperative to accurately identify the type of diabetes patients have, how long they’ve had diabetes, their diabetes complications , and their treatment priorities.
Older adults with diabetes are more likely to have health problems related to diabetes, such as chronic kidney disease, congestive heart failure, and stroke. They also may have memory and cognitive challenges, higher rates of functional and physical issues, and other health problems such as geriatric syndromes. Unique features of known health conditions like incontinence and frailty also occur in older patients. In addition, older adults may need more caregiver support.
Q: How do physical activity, following healthy eating patterns, adequate sleep, and social support change as adults with diabetes become older?
A: Getting enough sleep and regular physical activity and consuming healthy foods and drinks are crucial for managing blood glucose levels, preserving muscle strength, and preventing diabetes health problems. Older adults with diabetes may face limits to mobility, such as joint pain and fear of falling. They may have to follow multiple dietary guidelines if they have other health problems, which could result in more restricted eating habits. Certain medicines they take could interact with foods or cause changes in appetite, which also affects diet. Older adults may also have dental issues that restrict what they are able to eat. Having social connections with available family, friends, or neighbors is also important for helping older adults manage their diabetes.
In the Lifestyle Intervention for Seniors with Diabetes (LISD) study, we found that a lifestyle intervention strategy is highly successful in improving the diabetes management and physical function of older adults with diabetes. It may never be too late in life to begin lifestyle changes that include healthy eating habits and regular physical activity. Lifestyle changes can complement or even reduce the need for medical therapy. We also found that lifestyle interventions directly counter the increase in body fat and lack of physical activity that are primarily responsible for the age-related rise in insulin resistance that leads to type 2 diabetes.
Q: What are the unique challenges in managing blood glucose levels for older adults with diabetes? How can we balance the need for meeting blood glucose level targets while avoiding hypoglycemia and its associated risks?
A: There are certainly greater risks for hypoglycemia in older adults due to polypharmacy—or managing multiple medicines that might interact with each other. The signs of hypoglycemia might also be misread as neurologic symptoms of dementia. Health care professionals should tailor diabetes treatment using medicines that are less likely to cause low blood glucose and are proven safe or protective for the heart.
Medicines such as sulfonylureas, for example, have not been proven safe for the heart. They might also increase the risk for hypoglycemia because they are insulin secretagogues, which stimulate insulin release. Medicines such as the SGLT2 inhibitors, GLP-1 agonists, and metformin are less likely to cause hypoglycemia.
Q: What are ways that health care professionals can help patients address potential medicine interactions and simplify medicine regimens?
A: Health care professionals should regularly review the number of medicines their patients are taking to identify potential interactions. They should also consider reducing the number of medicines prescribed whenever possible, which may help reduce too many medicines or ones that aren’t necessary. Enlisting the expertise of pharmacists to review medicines can be very helpful, especially during transitions of care.
Health care professionals might also consider tools, such as reminder applications that can help patients track their medicines and take them as prescribed. Using the information from electronic health records can also help health care professionals manage their patients’ medicines. Finally, simplify their medicine regimens and instructions as much as possible, and do regular follow ups.
Q: How can the growing role of technology affect outcomes for older adults with diabetes? What are the barriers?
A: Technologies such as continuous glucose monitors (CGMs), insulin pumps, and smart pens can empower older adults with diabetes and their caregivers. These technologies may reduce complications and enhance quality of life. Many older adults with diabetes, especially if they take insulin, use CGMs. That’s straightforward, but they do need to work with their diabetes care team to learn how to take full advantage of CGMs to change health behaviors and adjust insulin doses. Insulin pumps could be challenging for older adults with diabetes who have cognitive problems. For example, older adults with cognitive problems may not remember all the steps to change tubing and cannula or give repeat insulin boluses.
Q: Are there specific social determinants of health barriers for older adults with diabetes that affect access to care? How can we address these barriers?
A: Racial disparities, social barriers, housing and food insecurity, transportation challenges, and isolation can all affect access to care as well as overall wellbeing. Health care professionals, regardless of their own background, should acknowledge these disparities and provide culturally competent care—which is care that meets the social, cultural, and language needs of their older adult patients with diabetes.
Addressing these barriers needs to be a comprehensive and multidisciplinary approach. So, we need to involve social workers, diabetes educators, and others who are part of health care teams.
Q: What lessons are we learning from research on managing the care and treatment of older adults with diabetes? Have we identified areas for future research?
A: Current research shows that older adults with diabetes are a varied group with many different types of people. We need to tailor care and treatment according to their health status. Some patients could be relatively healthy, others might be less healthy, and still others might have trouble with physical tasks or thinking skills. So, the goals should be to improve the quality of life. Some of these goals could include reducing the chance of having to go to the hospital and managing problems that come with getting older, such as falling and getting weaker.
We’ve learned that relaxing the target goal for managing blood glucose might be more important in people who have many other health problems. We also know that it’s key to regularly review medicines, reduce them as much as possible, and engage help from pharmacists and social workers.
Finally, culturally competent care is a challenge and should be a topic of more investigation. This is a promising area that could empower older adults with diabetes—particularly in a context of technology—and improve the quality of care. In addressing racial disparities, we need to identify culturally and clinically diverse treatments for older adults with diabetes. We also need to pinpoint the specific subgroups and care settings where diabetes technologies can be most effectively implemented.
What have you learned about managing the care of older adults with diabetes? Share below in the comments.
Subscribe to get blog updates.
Share this page
We welcome comments; all comments must follow our comment policy .
Blog posts written by individuals from outside the government may be owned by the writer and graphics may be owned by their creator. In such cases, it is necessary to contact the writer, artist, or publisher to obtain permission for reuse.
This Diabetes Drug Could Lower Your Risk Of Dementia, New Research Says
It's the 'gold standard' in treating congestive heart failure, too.

There has been a lot of chatter over the past year about the positive impacts of diabetes medications—like Ozempic —on a slew of serious health conditions . Now, there’s a new type of prescription joining the conversation—and it may lower your chances of getting dementia .
A recent study found that a class of medications called SGLT-2 inhibitors—which does not include Ozempic or similar drugs like Zepbound —significantly lowered the risk of dementia in people with type 2 diabetes.
Taking a diabetes medication to lower your risk of dementia seems like a big leap, but people with the condition are already at a higher risk of developing it. Here’s what you need to know about the latest findings, plus what’s behind this link.
Meet the experts : Pouya Shafipour, MD , a board-certified family and obesity medicine physician at Providence Saint John’s Health Center in Santa Monica, California. Jamie Alan, PhD , an associate professor of pharmacology and toxicology at Michigan State University.
Why are people with diabetes at a higher risk of dementia?
There is a known link between type 2 diabetes and dementia, with data suggesting that people with type 2 diabetes have a 50 percent greater risk of losing cognitive function. The association is most strongly tied to vascular dementia, which refers to "changes to memory, thinking, and behavior resulting from conditions that affect the blood vessels in the brain," per the National Institute on Aging .
This link is still under investigation, according to the American Heart Association (AHA). But people with type 2 diabetes seem to be at a higher risk if their blood sugar isn’t well-managed.
Research suggests that having too much glucose in the body may increase production of beta-amyloid, which proteins that clump together to form plaques in the brain that have been linked to Alzheimer's disease.
What did the study find?
The study, which was published in BMJ on August 28, analyzed data from the Korean National Health Insurance Service database, which included 110,885 people with type 2 diabetes between the ages of 40 and 69. All of those patients were either taking oral diabetes medications SGLT-2 or DPP-4 inhibitors for their condition. SGLT-2 helps the kidneys remove excess glucose from the body through urine, while DPP-4 works by deactivating the two main hormones that regulate blood sugar levels.
After 670 days, there were 1,172 new diagnoses of dementia among the study participants. When compared with people who took DPP-4 inhibitors, those who took SGLT-2 inhibitors had a 35 percent lower chance of developing dementia. Not only that, they had a 52 percent lower risk of vascular dementia, and a 39 percent lower risk of Alzheimer’s dementia.
People who took SGLT-2 inhibitors for longer periods of time seemed to have the best results. “SGLT-2 inhibitors might prevent dementia, providing greater benefits with longer treatment,” the researchers concluded in the study.
How do SGLT2 inhibitors work?
SGLT-2 inhibitors are a class of medications that treat type 2 diabetes. The medications help keep the kidneys from reabsorbing too much glucose and allow you to pee it out instead of circulating that glucose back into your blood, Alan explains.
“They are on the gold standard treatment list for congestive heart failure, too,” she says.
Why did the drug lower dementia risk?
This study didn’t explore why people had a lower risk of dementia on SGLT-2 inhibitors—it simply found a link. However, it may simply be that people on these medications had better blood sugar control, says Pouya Shafipour, MD , a board-certified family and obesity medicine physician at Providence Saint John’s Health Center in Santa Monica, California.
“Anything that stabilizes or lowers blood sugar and lowers a prolonged hyperglycemic state will help with this,” he says. “SGLT-2 has been found to be very helpful with that.” (Hyperglycemic means high blood sugar, in case you're not familiar with the term.)
Jamie Alan, PhD , an associate professor of pharmacology and toxicology at Michigan State University, agrees. “Lowering blood sugar would theoretically help dementia, particularly vascular dementia,” she says. “However, there is a lot about these drugs that we don’t know.”
Do other diabetes drugs (like Ozempic) have a similar effect?
It’s not clear yet. However, initial research conducted on semaglutide (the active ingredient in Ozempic) and other forms of GLP-1 drugs (the class of medications that Ozempic belongs to) has found that they may lower the risk of dementia.
One study published in December 2022 found that semaglutide lowered the risk of dementia in patients with type 2 diabetes. Another study found that fellow GLP-1 medication liraglutide may protect against dementia.
Are the results applicable to Americans?
This particular study was conducted on people in Korea—not America—and those are two different populations of people. However, experts say these results will likely apply to Americans, too.
How can people with diabetes lower their dementia risk?
It’s too soon to say that you should start taking an SGLT-2 inhibitor to lower your risk of dementia if you have diabetes. However, doing your best to control your blood sugar is often your best bet for lowering your risk of dementia, Shafipour says. That can include doing things like exercising regularly , eating a healthy diet that’s lower in refined carbohydrates, getting enough sleep , and doing your best to manage your stress , he says.
And, if you’re doing all of that and still struggling with blood sugar management, it may be time to rope in your doctor to talk about next steps.
How to Stay Safe During The Deli Meat Recall

Why Being Flexible May Help You Live Longer

How To Tell If You Have ‘Popcorn Brain’ Or ADHD

Best Electric Razors For Women In 2024, Per Derms

These Herbal Supplements Linked To Liver Damage

Learned Helplessness Can Hold You Back In Life

All About Kamala Harris' Diet And Workouts

How To Use Menstrual Cups And Discs, Per Experts

A Beginner’s Guide To The ‘Hara Hachi Bu’ Method

How To Do A Breast Self-Exam At Home, Per MDs

The Best Daily Supplements For Women Over 50

Orthorexia, A Form Of Disordered Eating, Is Rising
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmacological Reviews

New Aspects of Diabetes Research and Therapeutic Development
Both type 1 and type 2 diabetes mellitus are advancing at exponential rates, placing significant burdens on health care networks worldwide. Although traditional pharmacologic therapies such as insulin and oral antidiabetic stalwarts like metformin and the sulfonylureas continue to be used, newer drugs are now on the market targeting novel blood glucose–lowering pathways. Furthermore, exciting new developments in the understanding of beta cell and islet biology are driving the potential for treatments targeting incretin action, islet transplantation with new methods for immunologic protection, and the generation of functional beta cells from stem cells. Here we discuss the mechanistic details underlying past, present, and future diabetes therapies and evaluate their potential to treat and possibly reverse type 1 and 2 diabetes in humans.
Significance Statement
Diabetes mellitus has reached epidemic proportions in the developed and developing world alike. As the last several years have seen many new developments in the field, a new and up to date review of these advances and their careful evaluation will help both clinical and research diabetologists to better understand where the field is currently heading.
I. Introduction
Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020 ). Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing pancreatic beta cells. The much more prevalent T2D arises in conjunction with peripheral tissue insulin resistance and beta cell failure and is estimated to increase to 21%–33% of the US population by the year 2050 (Boyle et al., 2010 ). To combat this growing health threat and its cardiac, renal, and neurologic comorbidities, new and more effective diabetes drugs and treatments are essential. As the last several years have seen many new developments in the field of diabetes pharmacology and therapy, we determined that a new and up to date review of these advances was in order. Our aim is to provide a careful evaluation of both old and new therapies ( Fig. 1 ) in a manner that we hope will be of interest to both clinical and bench diabetologists. Instead of the usual encyclopedic approach to this topic, we provide here a targeted and selective consideration of the underlying issues, promising new treatments, and a re-examination of more traditional approaches. Thus, we do not discuss less frequently used diabetes agents, such as alpha-glucosidase inhibitors; these were discussed in other recent reviews (Hedrington and Davis, 2019 ; Lebovitz, 2019 ).

Pharmacologic targeting of numerous organ systems for the treatment of diabetes. Treatment of diabetes involves targeting of various organ systems, including the kidney by SGLT2 inhibitors; the liver, gut, and adipose tissue by metformin; and direct actions upon the pancreatic beta cell. Beta cell compounds aim to increase secretion or mass and/or to protect from autoimmunity destruction. Ultimately, insulin therapy remains the final line of diabetes treatment with new technologies under development to more tightly regulate blood glucose levels similar to healthy beta cells. hESC, human embryonic stem cell.
II. Diabetes Therapies
A. metformin.
Metformin is a biguanide originally based on the natural product galegine, which was extracted from the French lilac (Bailey, 1992 ; Rojas and Gomes, 2013 ; Witters, 2001 ). A closely related biguanide, phenformin, was also used initially for its hypoglycemic actions. Based on its successful track record as a safe, effective, and inexpensive oral medication, metformin has become the most widely prescribed oral agent in the world in treating T2D (Rojas and Gomes, 2013 ; He and Wondisford, 2015 ; Witters, 2001 ), whereas phenformin has been largely bypassed due to its unacceptably high association with lactic acidosis (Misbin, 2004 ). Unlike sulfonylureas, metformin lowers blood glucose without provoking hypoglycemia and improves insulin sensitivity (Bailey, 1992 ). Despite these well known beneficial metabolic actions, metformin’s mechanism of action and even its main target organ remain controversial. In fact, metformin has multiple mechanisms of action at the organ as well as the cellular level, which has hindered our understanding of its most important molecular effects on glucose metabolism (Witters, 2001 ). Adding to this, a specific receptor for metformin has never been identified. Metformin has actions on several tissues, although the primary foci of most studies have been the liver, skeletal muscle, and the intestine (Foretz et al., 2014 ; Rena et al., 2017 ). Metformin and phenformin clearly suppress hepatic glucose production and gluconeogenesis, and they improve insulin sensitivity in the liver and elsewhere (Bailey, 1992 ). The hepatic actions of metformin have been the most exhaustively studied to date, and there is little doubt that these actions are of some importance. However, several of the studies remain highly controversial, and there are still open questions.
One of the first reported specific molecular targets of metformin was mitochondrial complex I of the electron transport chain. Inhibition of this complex results in reduced oxidative phosphorylation and consequently decreased hepatic ATP production (El-Mir et al., 2008 ; Evans et al., 2005 ; Owen et al., 2000 ). As is the case in many other studies of metformin, however, high concentrations of the drug were found to be necessary to depress metabolism at this site (El-Mir et al., 2000 ; He and Wondisford, 2015 ; Owen et al., 2000 ). Also controversial is whether metformin works by activating 5′ AMP-activated protein kinase (AMPK), a molecular energy sensor that is known to be a major metabolic sensor in cells, or if not AMPK directly, then one of its upstream regulators such as liver kinase B2 (Zhou et al., 2001 ). Although metformin was shown to activate AMPK in several excellent studies, other studies directly contradicted the AMPK hypothesis. Most dramatic were studies showing that metformin’s actions to suppress hepatic gluconeogenesis persisted despite genetic deletion of the AMPK’s catalytic domain (Foretz et al., 2010 ). More recent studies identified additional or alternative targets, such as cAMP signaling in the liver (Miller et al., 2013 ) or glycogen synthase kinase-3 (Link, 2003 ). Other work showed that the phosphorylation of acetyl-CoA carboxylase and acetyl-CoA carboxylase 2 are involved in regulating lipid homeostasis and improving insulin sensitivity after exposure to metformin (Fullerton et al., 2013 ).
Although there are strong data to support each of these pathways, it is not entirely clear which signaling pathway(s) is most essential to the actions of metformin in hepatocytes. Metformin clearly inhibits complex I and concomitantly decreases ATP and increases AMP. The latter results in AMPK activation, reduced fatty acid synthesis, and improved insulin receptor activation, and increased AMP has been shown to inhibit adenylate cyclase to reduce cAMP and thus protein kinase A activation. Downstream, this reduces the expression of phosphoenolpyruvate carboxykinase and glucose 6-phosphatase via decreased cAMP response element-binding protein, the cAMP-sensitive transcription factor. Decreased PKA also promotes ATP-dependent 6-phosphofructokinase, liver type activity via fructose 2,6-bisphosphate and reduces gluconeogenesis, as fructose-bisphosphatase 1 is inhibited by fructose 2,6-bisphosphate, along with other mechanisms (Rena et al., 2017 ; Pernicova and Korbonits, 2014 ).
More recent work has shown that metformin at pharmacological rather than suprapharmacological doses increases mitochondrial respiration and complex 1 activity and also increases mitochondrial fission, now thought to be critical for maintaining proper mitochondrial density in hepatocytes and other cells. This improvement in respiratory activity occurs via AMPK activation (Wang et al., 2019 ).
Although the liver has historically been the major suspected site of metformin action, recent studies have suggested that the gut instead of the liver is a major target, a concept supported by the increased efficacy of extended-release formulations of metformin that reside for a longer duration in the gut after their administration (Buse et al., 2016 ). An older, but in our view an important observation, is that the intravenous administration of metformin has little or no effect on blood glucose, whereas, in contrast, orally administered metformin is much more effective (Bonora et al., 1984 ). Recent imaging studies using labeled glucose have shown directly that metformin stimulates glucose uptake by the gut in patients with T2D to reduce plasma glucose concentrations (Koffert et al., 2017 ; Massollo et al., 2013 ). Additionally, it is possible that metformin may exert its effect in the gut by inducing intestinal glucagon-like peptide-1 (GLP-1) release (Mulherin et al., 2011 ; Preiss et al., 2017) to potentiate beta cell insulin secretion and by stimulating the central nervous system (CNS) to exert control over both blood glucose and liver function. Indeed, CNS effects produced by metformin have been proposed to occur via the local release of GLP-1 to activate intestinal nerve endings of ascending nerve pathways that are involved in CNS glucose regulation (Duca et al., 2015 ). Lastly, several papers have now implicated that metformin may act by altering the gut microbiome, suggesting that changes in gut flora may be critical for metformin’s actions (McCreight et al., 2016 ; Wu et al., 2017 ; Devaraj et al., 2016 ). A new study proposed that activation of the intestinal farnesoid X receptor may be the means by which microbiota alter hyperglycemia (Sun et al., 2018 ). However, these studies will require more mechanistic detail and confirmation before they can be fully accepted by the field. In addition to the action of metformin on gut flora, the production of imidazole propionate by gut microbes in turn has been shown to interfere with metformin action through a p38-dependent mechanism and AMPK inhibition. Levels of imidazole propionate are especially higher in patients with T2D who are treated with metformin (Koh et al., 2020 ).
In summary, the combined contribution of these various effects of metformin on multiple cellular targets residing in many tissues may be key to the benefits of metformin treatment on lowering blood glucose in patients with type 2 diabetes (Foretz et al., 2019 ). In contrast, exciting new work showing metformin leads to weight loss by increasing circulating levels of the peptide hormone growth differentiation factor 15 and activation of brainstem glial cell-derived neurotropic factor family receptor alpha like receptors to reduce food intake and energy expenditure works independently of metformin’s glucose-lowering effect (Coll et al., 2020 ).
B. Sulfonylureas and Beta Cell Burnout
The class of compounds known as sulfonylureas includes one of the oldest oral antidiabetic drugs in the pharmacopoeia: tolbutamide. Tolbutamide is a “first generation” oral sulfonylurea secretagogue whose clinical usefulness is due to its prompt stimulation of insulin release from pancreatic beta cells. “Second generation” sulfonylureas include drugs such as glyburide, gliclazide, and glipizide. Sulfonylureas act by binding to a high affinity sulfonylurea binding site, the sulfonylurea receptor 1 subunit of the K(ATP) channel, which closes the channel. These drugs mimic the physiologic effects of glucose, which closes the K(ATP) channel by raising cytosolic ATP/ADP. This in turn provokes beta cell depolarization, resulting in increased Ca 2+ influx into the beta cell (Ozanne et al., 1995 ; Ashcroft and Rorsman, 1989 ; Nichols, 2006 ). Importantly, sulfonylureas, and all drugs that directly increase insulin secretion, are associated with hypoglycemia, which can be severe, and which limits their widespread use in the clinic (Yu et al., 2018 ). Meglitinides are another class of oral insulin secretagogues that, like the sulfonylureas, bind to sulfonylurea receptor 1 and inhibit K(ATP) channel activity (although at a different site of action). The rapid kinetics of the meglitinides enable them to effectively blunt the postprandial glycemic excursions that are a hallmark (along with elevated fasting glucose) of T2D (Rosenstock et al., 2004). However, the need for their frequent dosing (e.g., administration before each meal) has limited their appeal to patients.
The efficacy of sulfonylureas is known to decrease over time, leading to failure of the class for effective long-term treatment of T2D (Harrower, 1991 ). More broadly, it is now widely accepted that the number of functional beta cells in humans declines during the progression of T2D. Thus, one would expect that due to this decline, all manner of oral agents intended to target the beta cell and increase its cell function (and especially insulin secretion) will fail over time (RISE Consortium, 2019 ), a process referred to as “beta cell failure” (Prentki and Nolan, 2006 ). Currently, treatments that can expand beta cell mass or improve beta cell function or survival over time are not yet available for use in the clinic. As a result, treatments that may be able to help patients cope with beta cell burnout such as islet cell transplantation, insulin pumps, or stem cell therapy are alternatives that will be discussed below.
C. Ca 2+ Channel Blockers and Type 1 Diabetes
Strategies to treat and prevent T1D have historically focused on ameliorating the toxic consequences of immune dysregulation resulting in autoimmune destruction of pancreatic beta cells. More recently, a concerted focus on alleviating the intrinsic beta cell defects (Sims et al., 2020 ; Soleimanpour and Stoffers, 2013 ) that also contribute to T1D pathogenesis have been gaining traction at both the bench and the bedside. Several recent preclinical studies suggest that Ca 2+ -induced metabolic overload induces beta cell failure (Osipovich et al., 2020 ; Stancill et al., 2017 ; Xu et al., 2012 ), with the potential that excitotoxicity contributes to beta cell demise in both T1D and T2D, similar to the well known connection between excitotoxicity and, concomitantly, increased Ca 2+ loading of the cells and neuronal dysfunction. Indeed, the use of the phenylalkylamine Ca 2+ channel blocker verapamil has been successful in ameliorating beta cell dysfunction in preclinical models of both T1D and T2D (Stancill et al., 2017 ; Xu et al., 2012 ). Verapamil is a well known blocker of L-type Ca 2+ channels, and, in normally activated beta cells, it limits Ca 2+ entry into the beta cell (Ohnishi and Endo, 1981 ; Vasseur et al., 1987 ). This would be expected to, in turn, alter the expression of many Ca 2+ influx–dependent beta cell genes (Stancill et al., 2017 ), and the evidence to date suggests it is likely that verapamil preserves beta cell function in diabetes models by repressing thioredoxin-interacting protein (TXNIP) expression and thus protecting the beta cell. This is somewhat surprising given the physiologic role of Ca 2+ is to acutely trigger insulin secretion; this process would be expected to be inhibited by L-type Ca 2+ channel blockers (Ashcroft and Rorsman, 1989 ; Satin et al., 1995 ).
Hyperglycemia is a well known inducer of TXNIP expression, and a lack of TXNIP has been shown to protect against beta cell apoptosis after inflammatory stress (Chen et al., 2008a ; Shalev et al., 2002 ; Chen et al., 2008b ). Excitingly, the use of verapamil in patients with recent-onset T1D improved beta cell function and improved glycemic control for up to 12 months after the initiation of therapy, suggesting there is indeed promise for targeting calcium and TXNIP activation in T1D. Use of verapamil for a repurposed indication in the preservation of beta cell function in T1D is attractive due its well known safety profile as well as its cardiac benefits (Chen et al., 2009 ). Although the long-term efficacy of verapamil to maintain beta cell function in vivo is unclear, a recently described TXNIP inhibitor may also show promise in suppressing the hyperglucagonemia that also contributes to glucose intolerance in T2D (Thielen et al., 2020 ). As there is a clear need for increased Ca 2+ influx into the beta cell to trigger and maintain glucose-dependent insulin secretion (Ashcroft and Rorsman, 1990 ; Satin et al., 1995 ), it remains to be seen how well regulated insulin secretion is preserved in the presence of L-type Ca 2+ channel blockers like verapamil in the system. One might speculate that reducing but not fully eliminating beta cell Ca 2+ influx might reduce TXNIP levels while preserving enough influx to maintain glucose-stimulated insulin release. Alternatively, these two phenomena may operate on entirely different time scales. At present, these issues clearly will require further investigation.
D. GLP-1 and the Incretins
Studies dating back to the 1960s revealed that administering glucose in equal amounts via the peripheral circulation versus the gastrointestinal tract led to dramatically different amounts of glucose-induced insulin secretion (Elrick et al., 1964 ; McIntyre et al., 1964 ; Perley and Kipnis, 1967 ). Gastrointestinal glucose administration greatly increased insulin secretion versus intravenous glucose, and this came to be known as the “incretin effect” (Nauck et al., 1986a ; Nauck et al., 1986b ). Subsequent work showed that release of the gut hormone GLP-1 mediated this effect such that food ingestion induced intestinal cell hormone secretion. GLP-1 so released would then circulate to the pancreas via the blood to prime beta cells to secrete more insulin when glucose became elevated because these hormones stimulated beta cell cAMP formation (Drucker et al., 1987 ). The discovery that a natural peptide corresponding to GLP-1 could be found in the saliva of the Gila monster, a desert lizard, hastened progress in the field, and ample in vitro studies subsequently confirmed that GLP-1 potentiated insulin secretion in a glucose-dependent manner. GLP-1 has little or no significant action on insulin secretion in the absence of elevated glucose (such as might typically correspond to the postprandial case or during fasting), thus minimizing the likelihood of hypoglycemia provoked by GLP-1 in treated patients (Kreymann et al., 1987 ). Although not completely understood, the glucose dependence of GLP-1 likely reflects the requirement for adenine nucleotides to close glucose-inhibited K(ATP) channels and thus subsequently activate Ca 2+ influx–dependent insulin exocytosis. Besides potentiating GSIS at the level of the beta cell, glucagon-like peptide-1 receptor (GLP-1R) agonists also decrease glucagon secretion from pancreatic islet alpha cells, reduce gastric emptying, and may also increase beta cell proliferation, among other cellular actions (reviewed in Drucker, 2018 ; Muller et al., 2019).
Intense interest in the incretins by basic scientists, clinicians, and the pharma community led to the rapid development of new drugs for treating primarily T2D. These drugs include a range of GLP-1R agonists and inhibitors of the incretin hormone degrading enzyme dipeptidyl peptidase 4 (DPP4), whose targeting increases the half-lives of GLP-1 and gastric inhibitory polypeptide (GIP) and thereby increases protein hormone levels in plasma. GLP-1R agonists have been associated with not only a lowering of plasma glucose but also weight loss, decreased appetite, reduced risk of cardiovascular events, and other favorable outcomes (Gerstein et al., 2019; Hernandez et al., 2018; Husain et al., 2019; Marso et al., 2016a; Marso et al., 2016b ; Buse et al., 2004). Regarding their untoward actions, although hypoglycemia is not a major concern, there have been reports of pancreatitis and pancreatic cancer from use of GLP-1R agonists. However, a recent meta-analysis covering four large-scale clinical trials and over 33,000 participants noted no significantly increased risk for pancreatitis/pancreatic cancer in patients using GLP-1R agonists (Bethel et al., 2018).
Ongoing and future developments in the use of proglucagon-derived peptides such as GLP-1 and glucagon include the use of combined GLP-1/GIP, glucagon/GLP-1, and agents targeting all three peptides in combination (reviewed in Alexiadou and Tan, 2020 ). Although short-term infusions of GLP-1 with GIP failed to yield metabolic benefits beyond those seen with GLP-1 alone (Bergmann et al., 2019 ), several GLP-1/GIP dual agonists are currently in development and have shown promising metabolic results in clinical trials (Frias et al., 2017 ; Frias et al., 2020 ; Frias et al., 2018 ). At the level of the pancreatic islet, beneficial effects of dual GLP-1/GIP agonists may be related to imbalanced and biased preferences of these agonists for the gastric inhibitory polypeptide receptor over the GLP-1R (Willard et al., 2020 ) and possibly were not simply to dual hormone agonism in parallel. Dual glucagon/GLP-1 agonist therapy has also been shown to have promising metabolic effects in humans (Ambery et al., 2018 ; Tillner et al., 2019 ). Oxyntomodulin is a natural dual glucagon/GLP-1 receptor agonist and proglucagon cleavage product that is also secreted from intestinal enteroendocrine cells, which has beneficial effects on insulin secretion, appetite regulation, and body weight in both humans and rodents (Cohen et al., 2003 ; Dakin et al., 2001 ; Dakin et al., 2002 ; Shankar et al., 2018 ; Wynne et al., 2005 ). Interestingly, alpha cell crosstalk to beta cells through the combined effects of glucagon and GLP-1 is necessary to obtain optimal glycemic control, suggesting a potential pathway for therapeutic dual glucagon/GLP-1 agonism within the islets of patients with T2D (Capozzi et al., 2019a ; Capozzi et al., 2019b ). Although the early results appear promising, more studies will be necessary to better understand the mechanistic and clinical impacts of these multiagonist agents.
E. DPP4 Inhibitors
Inhibition of DPP4, the incretin hormone degrading enzyme, is one of the most common T2D treatments to increase GLP-1 and GIP plasma hormone levels. These DPP4 inhibitors or “gliptins” are generally used in conjunction with other T2D drugs such as metformin or sulfonylureas to obtain the positive benefits discussed above (Lambeir et al., 2008 ). DPP4 is a primarily membrane-bound peptidase belonging to the serine peptidase/prolyl oligopeptidase gene family, which cleaves a large number of substrates in addition to the incretin hormones (Makrilakis, 2019 ). DPP4 inhibitors provide glucose-lowering benefits while being generally well tolerated, and the variety of available drugs (including sitagliptin, saxagliptin, vildagliptin, alogliptin, and linagliptin) with slightly different dosing frequency, half-life, and mode of excretion/metabolism allows for use in multiple patient populations (Makrilakis, 2019 ). This includes the elderly and individuals with renal or hepatic insufficiency (Makrilakis, 2019 ).
Although hypoglycemia is not a concern for DPP4 inhibitor use, other considerations should be made. DPP4 inhibitors tend to be more expensive than metformin or other second-line oral drugs in addition to having more modest glycemic effects than GLP-1R agonists (Munir and Lamos, 2017 ). Finally, meta-analysis of randomized and observational studies concluded that heart failure in patients with T2D was not associated with use of DPP4 inhibitors; however, this study was limited by the short follow-up and lack of high-quality data (Li et al., 2016 ). Thus, the US Food and Drug Administration (FDA) did recommend assessing risk of heart failure hospitalization in patients with pre-existing cardiovascular disease, prior heart failure, and chronic kidney disease when using saxagliptin and alogliptin (Munir and Lamos, 2017 ).
F. Sodium Glucose Cotransporter 2 Inhibitors
A recent development in the field of T2D drugs are sodium glucose cotransporter 2 (SGLT2) inhibitors, which have an interesting and very different mechanism of action. Within the proximal tubule of the nephron, SGLT2 transports ingested glucose into the lumen of the proximal tubule between the epithelial layers, thereby reclaiming glucose by this reabsorption process (reviewed in Vallon, 2015 ). SGLT2 inhibitors target this transporter and increase glucose in the tubular fluid and ultimately increase it in the urine. In patients with diabetes, SGLT2 inhibition results in a lowering of plasma glucose with urine glucose content rising substantially (Adachi et al., 2000 ; Vallon, 2015 ). These drugs, although they are relatively new, have become an area of great interest for not only patients with T2D (Grempler et al., 2012 ; Imamura et al., 2012 ; Meng et al., 2008 ; Nomura et al., 2010 ) but also for patients with T1D (Luippold et al., 2012 ; Mudaliar et al., 2012 ). Part of their appeal also rests on reports that their use can lead to a statistically significant decline in cardiac events that are known to occur secondarily to diabetes, possibly independently of plasma glucose regulation (reviewed in Kurosaki and Ogasawara, 2013 ). Although the long-term consequences of their clinical use cannot yet be determined, raising the glucose content of the urogenital tract leads to an increased risk of urinary tract infections and other related infections in some patients (Kurosaki and Ogasawara, 2013 ).
Another recent concern about the use of SGLT2 inhibitors has been the development of normoglycemic diabetic ketoacidosis (DKA). Despite the efficacy of SGLT2 inhibitors, observations of hyperglucagonemia in patients with euglycemic DKA has led to a number of recent studies focused on SGLT2 actions on pancreatic islets. Initial studies of isolated human islets treated with small interfering RNA directed against SGLT2 and/or SGLT2 inhibitors demonstrated increased glucagon release. These studies were complemented by the finding of elevations in glucagon release in mice that were administered SGLT2 inhibitors in vivo (Bonner et al., 2015 ). Insights into the possible mechanistic links between SGLT2 inhibition, DKA frequency, and glucagon secretion in humans may relate to the observation of heterogeneity in SGLT2 expression, as SGLT2 expression appears to have a high frequency of interdonor and intradonor variability (Saponaro et al., 2020 ). More recently, both insulin and GLP-1 have been demonstrated to modulate SGLT2-dependent glucagon release through effects on somatostatin release from delta cells (Vergari et al., 2019 ; Saponaro et al., 2019 ), suggesting potentially complex paracrine effects that may affect the efficacy of these compounds.
On the other hand, several recent studies question that the development of euglycemic DKA after SGLT2 inhibitor therapy may be through alpha cell–dependent mechanisms. Three recent studies found no effect of SGLT2 inhibitors to promote glucagon secretion in mouse and/or rat models and could not detect SGLT2 expression in human alpha cells (Chae et al., 2020 ; Kuhre et al., 2019 ; Suga et al., 2019 ). A fourth study demonstrated only a brief transient effect of SGLT2 inhibition to raise circulating glucagon concentrations in immunodeficient mice transplanted with human islets, which returned to baseline levels after longer exposures to SGLT2 inhibitors (Dai et al., 2020 ). Furthermore, SGLT2 protein levels were again undetectable in human islets (Dai et al., 2020 ). These results could suggest alternative islet-independent mechanisms by which patients develop DKA, including alterations in ketone generation and/or clearance, which underscore the additional need for further studies both in molecular models and at the bedside. Nevertheless, SGLT2 inhibitors continue to hold promise as a valuable therapy for T2D, especially in the large segment of patients who also have superimposed cardiovascular risk (McMurray et al., 2019; Wiviott et al., 2019; Zinman et al., 2015).
G. Thiazolidinediones
Once among the most commonly used oral agents in the armamentarium to treat T2D, thiazolidinediones (TZDs) were clinically popular in their utilization to act specifically as insulin sensitizers. TZDs improve peripheral insulin sensitivity through their action as peroxisome proliferator-activated receptor (PPAR) γ agonists, but their clinical use fell sharply after studies suggested a connection between cardiovascular toxicity with rosiglitazone and bladder cancer risk with pioglitazone (Lebovitz, 2019 ). Importantly, an FDA panel eventually removed restrictions related to cardiovascular risk with rosiglitazone in 2013 (Hiatt et al., 2013 ). Similarly, concerns regarding use of bladder cancer risk with pioglitazone were later abated after a series of large clinical studies found that pioglitazone did not increase bladder cancer (Lewis et al., 2015 ; Schwartz et al., 2015 ). However, usage of TZDs had already substantially decreased and has not since recovered.
Although concerns regarding edema, congestive heart failure, and fractures persist with TZD use, there have been several studies suggesting that TZDs protect beta cell function. In the ADOPT study, use of rosiglitazone monotherapy in patients newly diagnosed with T2D led to improved glycemic control compared with metformin or sulfonylureas (Kahn et al., 2006). Later analyses revealed that TZD-treated subjects had a slower deterioration of beta cell function than metformin- or sulfonylurea-treated subjects (Kahn et al., 2011). Furthermore, pioglitazone use improved beta cell function in the prevention of T2D in the ACT NOW study (Defronzo et al., 2013; Kahn et al., 2011). Mechanistically, it is unclear if TZDs lead to beneficial beta cell function through direct effects or through indirect effects of reduced beta cell demand due to enhanced peripheral insulin sensitivity. Indeed, a beta cell–specific knockout of PPAR γ did not impair glucose homeostasis, nor did it impair the antidiabetic effects of TZD use in mice (Rosen et al., 2003 ). However, other reports demonstrated PPAR-responsive elements within the promoters of both glucose transporter 2 and glucokinase that enhance beta cell glucose sensing and function, which could explain beta cell–specific benefits for TZDs (Kim et al., 2002 ; Kim et al., 2000 ). Furthermore, TZDs have been shown to improve beta cell function by upregulating cholesterol transport (Brunham et al., 2007 ; Sturek et al., 2010 ). Additionally, use of TZDs in the nonobese diabetic (NOD) mouse model of T1D augmented the beta cell unfolded protein response and prevented beta cell death, suggesting potential benefits for TZDs in both T1D and T2D (Evans-Molina et al., 2009 ; Maganti et al., 2016 ). With a now refined knowledge of demographics in which to avoid TZD treatment due to adverse effects, together with genetic approaches to identify candidates more likely to respond effectively to TZD therapy (Hu et al., 2019 ; Soccio et al., 2015 ), it remains to be seen if TZD therapy will return to more prominent use in the treatment of diabetes.
H. Insulin and Beyond: The Use of “Smart” Insulin and Closed Loop Systems in Diabetes Treatment
Due to recombinant DNA technology, numerous insulin analogs are now available in various forms ranging from fast acting crystalline insulin to insulin glargine; all of these analogs exhibit equally effective insulin receptor binding. Most are generated by altering amino acids in the B26–B30 region of the molecule (Kurtzhals et al., 2000 ). The American Diabetes Association delineates these insulins by their 1) onset or time before insulin reaches the blood stream, 2) peak time or duration of maximum blood glucose–lowering efficacy, and 3) the duration of blood glucose–lowering time. Insulin administration is independent of the residuum of surviving and/or functioning beta cells in the patient and remains the principal pharmacological treatment of both T1D and T2D. The availability of multiple types of delivery methods, i.e., insulin pens, syringes, pumps, and inhalants, provides clinicians with a solid and varied tool kit with which to treat diabetes. The downsides, however, are that 1) hypoglycemia is a constant threat, 2) proper insulin doses are not trivial to calculate, 3) compliance can vary especially in children and young adults, and 4) there can be side effects of a variety of types. Nonetheless, insulin therapy remains a mainstay treatment of diabetes.
To eliminate the downsides of insulin therapy, research in the past several decades has worked toward generating glucose-sensitive or “smart” insulin molecules. These molecules change insulin bioavailability and become active only upon high blood glucose using glucose-binding proteins such as concanavalin A, glucose oxidase to alter pH sensitivity, and phenylboronic acid (PBA), which forms reversible ester linkages with diol-containing molecules including glucose itself (reviewed in Rege et al., 2017 ). Indeed, promising recent studies included various PBA moieties covalently bonded to an acylated insulin analog (insulin detemir, which contains myristic acid coupled to Lys B29 ). The detemir allows for binding to serum albumin to prolong insulin’s half-life in the circulation, and PBA provided reversible glucose binding (Chou et al., 2015 ). The most promising of the PBA-modified conjugates showed higher potency and responsiveness in lowering blood glucose levels compared with native insulin in diabetic mouse models and decreased hypoglycemia in healthy mice, although the molecular mechanisms have not yet been determined (Chou et al., 2015 ).
An additional active area of research includes structurally defining the interaction between insulin and the insulin receptor ectodomain. Importantly, a major conformational change was discovered that may be exploited to impair insulin receptor binding under hypoglycemic conditions (Menting et al., 2013 ; Rege et al., 2017 ). Challenges in the design, testing, and execution of glucose-responsive insulins may be overcome by the adaptation of novel modeling approaches (Yang et al., 2020 ), which may allow for more rapid screening of candidate compounds.
Technologies have also progressed in the field of artificial pancreas design and development. Currently two “closed loop” systems are now available: Minimed 670G from Medtronic and Control-IQ from Tandem Diabetes Care. Both systems use a continuous glucose monitor, insulin pump, and computer algorithm to predict correct insulin doses and administer them in real time. Such algorithm systems also take into account insulin potency, the rate of blood glucose increase, and the patient’s heart rate and temperature to adjust insulin delivery levels during exercise and after a meal. In addition, so-called “artificial pancreas” systems have also been clinically tested, which use both insulin and glucagon and as such result in fewer reports of hypoglycemic episodes (El-Khatib et al., 2017 ). These types of systems will continue to become more popular as the development of room temperature–stable glucagon analogs continue, such as GVOKE by Xeris Pharmaceuticals (currently available in an injectable syringe) and Baqsimi, a nasally administered glucagon from Eli Lilly.
I. Present and Future Therapies: Beta Cell Transplantation, Replication, and Immune Protection
1. islet transplantation.
The idea to use pancreatic allo/xenografts to treat diabetes remarkably dates back to the late 1800s (Minkowski, 1892 ; Pybus, 1924 ; Williams, 1894 ). Before proceeding to the discovery of insulin (together with Best, MacLeod, and Collip), Frederick Banting also postulated the potential for transplantation of pancreatic tissue emulsions to treat diabetes in dog models in a notebook entry in 1921 (Bliss, 1982 ). Decades later, Paul Lacy, David Scharp, and colleagues successfully isolated intact functional pancreatic islets and transplanted them into rodent models (Kemp et al., 1973 ). These studies led to the initial proof of concept studies for humans, with the first successful islet transplant in a patient with T1D occurring in 1977 (Sutherland et al., 1978 ). A rapid expansion of islet transplantation, inspired by these original studies led to key observations of successfully prolonged islet engraftment by the “Edmonton protocol” whereby corticosteroid-sparing immunosuppression was applied, and islets from at least two allogeneic donors were used to achieve insulin independence (Shapiro et al., 2000 ). More recent work has focused on improving upon the efficiency and long-term engraftment of allogeneic transplants leading to more prolonged graft function (to the 5-year mark) and successful transplantation from a single islet donor (Hering et al., 2016; Hering et al., 2005 ; Rickels et al., 2013 ). Critical to these efforts to improve the success rate was the recognition that the earlier generation of immunosuppressive agents to counter tissue rejection was toxic to islets (Delaunay et al., 1997 ; Paty et al., 2002 ; Soleimanpour et al., 2010 ) and that more appropriate and less toxic agents were needed (Hirshberg et al., 2003 ; Soleimanpour et al., 2012 ).
Certainly, islet transplantation as a therapeutic approach for patients with T1D has been scrutinized due to several challenges, including (but not limited to) the lack of available donor supply to contend with demand, limited long-term functional efficacy of islet allografts, the potential for re-emergence of autoimmune islet destruction and/or metabolic overload-induced islet failure, and significant adverse effects of prolonged immunosuppression (Harlan, 2016 ). Furthermore, although islet transplantation is not currently available for individuals with T2D, simultaneous pancreas-kidney transplantation in T2D had similar favorable outcomes to simultaneous pancreas-kidney transplantation in T1D; therefore, islet-kidney transplantation may eventually be a feasible option to treat T2D, as patients will already be on immunosuppressors (Sampaio et al., 2011 ; Westerman et al., 1983 ). An additional significant obstacle is the tremendous expense associated with islet transplantation therapy. Indeed, the maintenance, operation, and utilization of an FDA-approved and Good Manufacturing Practice–compliant islet laboratory can lead to operating costs at nearly $150,000 per islet transplant, which is not cost effective for the vast majority of patients with T1D (Naftanel and Harlan, 2004 ; Wallner et al., 2016 ). At present, the focus has been to obtain FDA approval for islet allo-transplantation as a therapy for T1D to allow for insurance compensation (Hering et al., 2016; Rickels and Robertson, 2019 ). In the interim, the islet biology, stem cell, immunology, and bioengineering communities have continued the development of cell-based therapies for T1D by other approaches to overcome the challenges identified during the islet transplantation boom of the 1990s and 2000s.
2. Pharmacologic Induction of Beta Cell Replication
Besides transplantation, progress in islet cell biology and especially in developmental biology of beta cells over several decades raised the additional possibility that beta cell mass reduction in diabetes might be countered by increasing beta cell number through mitogenic means. A key method to expand pancreatic beta cell mass is through the enhancement of beta cell replication. Although the study of pancreatic beta cell replication has been an area of intense focus in the beta cell biology field for several decades, only recently has this seemed truly feasible. Seminal studies identified that human beta cells are essentially postmitotic, with a rapid phase of growth occurring in the prenatal period that dramatically tapers off shortly thereafter (Gregg et al., 2012 ; Meier et al., 2008 ). The plasticity of rodent beta cells is considerably higher than that of human beta cells (Dai et al., 2016 ), which has led to a renewed focus on validation of pharmacologic agents to enhance rodent beta cell replication using isolated and/or engrafted human islets (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). Indeed, a large percentage of agents that were successful when applied to rodent systems were largely unsuccessful at inducing replication in human beta cells (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). However, several recent studies have begun to make significant progress on successfully pushing human beta cells to replicate.
Several groups have reported successful human beta cell proliferation, both in vitro and in vivo, in response to inhibitors of the dual specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). These inhibitors include harmine, INDY, GNF4877, 5-iodotubericidin, leucettine-42, TG003, AZ191, CC-401, and more specific, recently developed DYRK1A inhibitors (Ackeifi et al., 2020 ). Although DYRK1A is conclusively established as the important mediator of human beta cell proliferation, comprehensively determining other cellular targets and if additional gene inhibition amplifies the proliferative response is still in process. New evidence from Wang and Stewart shows dual specificity tyrosine phosphorylation-regulated kinase 1B to be an additional mitogenic target and also describes variability in the range of activated kinases within cells and/or levels of inhibition for the many DYRK1A inhibitors listed above (Ackeifi et al., 2020 ). Interestingly, opposite to these human studies, earlier mouse studies from the Scharfmann group demonstrated that Dyrk1a haploinsufficiency leads to decreased proliferation and loss of beta cell mass (Rachdi et al., 2014b ). In addition, overexpression of Dyrk1a in mice led to beta cell mass expansion with increased glucose tolerance (Rachdi et al., 2014a ).
Although important differences in beta cell proliferative capacity have been shown between human and rodent species, there are also significant differences in the mitogenic capacity of beta cells from juvenile, adult, and pregnant individuals. This demonstrates that proliferative stimuli appear to act within the complex islet, pancreas, and whole-body environments unique to each time point. For example, the administration of the hormones platelet-derived growth factor alpha or GLP-1 result in enhanced proliferation in juvenile human beta cells yet are ineffective in adult human beta cells (Chen et al., 2011 ; Dai et al., 2017 ). This has been shown to be due to a loss of platelet-derived growth factor alpha receptor expression as beta cells age but appears to be unrelated to GLP-1 receptor expression levels (Chen et al., 2011 ). Indeed, the GLP-1 receptor is highly expressed in adult beta cells, and GLP-1 secretion increases insulin secretion, as detailed previously; however, the induction of proliferative factors such as nuclear factor of activated T cells, cytoplasmic 1; forkhead box protein 1; and cyclin A1 is only seen in juvenile islets (Dai et al., 2017 ). Human studies using cadaveric pancreata from pregnant donors also showed increased beta cell mass, yet lactogenic hormones from the pituitary or placenta (prolactin, placental lactogen, or growth hormone) are unable to stimulate proliferation in human beta cells despite their ability to produce robust proliferation in mouse beta cells (reviewed in Baeyens et al., 2016 ). Experiments overexpressing mouse versus human signal transducer and activator of transcription 5, the final signaling factor inducing beta cell adaptation, in human beta cells allows for prolactin-mediated proliferation revealing fundamental differences in prolactin pathway competency in human (Chen et al., 2015 ). Overcoming the barrier of recapitulating human pregnancy’s effect on beta cells through isolating placental cells or blood serum during pregnancy may result in the discovery of a factor(s) that facilitates the increase in beta cell mass observed during human pregnancy.
Mechanisms that stimulate beta cell proliferation have also been discovered from studying genetic mutations that result in insulinomas, spontaneous insulin-producing beta cell adenomas. The most common hereditary mutation occurs in the multiple endocrine neoplasia type 1 (MEN1) gene. Indeed, administration of a MEN1 inhibitor in addition to a GLP-1 agonist (which cannot induce proliferation alone) is able to increase beta cell proliferation in isolated human islets through synergistic activation of KRAS proto-oncogene, GTPase downstream signals (Chamberlain et al., 2014 ). Interestingly, MEN1 mutations are uncommon in sporadic insulinomas, yet assaying genomic and epigenetic changes in a large cohort of non-MEN1 insulinomas found alterations in trithorax and polycomb chromatin modifying genes that were functionally related to MEN1 (Wang et al., 2017 ). Stewart and colleagues hypothesized that changes in histone 3 lysine 27 and histone 3 lysine 4 methylation status led to increased enhancer of zeste homolog 2 and lysine demethylase 6A, decreased cyclin-dependent kinase inhibitor 1C, and thereby increased beta cell proliferation, among other phenotypes. They also proposed that these findings help to explain why increased proliferation always occurs despite broad heterogeneity of mutations found between individual insulinomas (Wang et al., 2017 ).
Although factors that induce proliferation are continuing to be discovered, there are drawbacks that still limit their clinical application. Harmine and other DYRK1A inhibitors are not beta cell specific, nor have all their cellular targets been determined (Ackeifi et al., 2020 ). Targeting other pathways to induce human beta cell proliferation such as modulation of prostaglandin E2 receptors (i.e., inhibition of prostaglandin E receptor 3 alone or in combination with prostaglandin E receptor 4 activation) showed promising increases in proliferative rate yet suffers from the same lack of specificity (Carboneau et al., 2017 ). Induction of proliferation may also come at the expense of glucose sensing as in insulinomas, which have an increased expression of “disallowed genes” and alterations in glucose transporter and hexokinase expression (Wang et al., 2017 ). A further untoward consequence that must be avoided is the production of cancerous cells through unchecked proliferation. Finally, increasing beta cell mass through low rates of proliferation may increase the pool of functional insulin-secreting cells in T2D, but without additional measures, these beta cells will still ultimately be targeted for immune cell destruction in T1D.
3. Beta Cell Stress Relieving Therapies
Metabolic, inflammatory, and endoplasmic reticulum (ER) stress contribute to beta cell dysfunction and failure in both T1D and T2D. Although reduction of metabolic overload of beta cells by early exogenous insulin therapy or insulin sensitizers can temporarily reduce loss of beta cell mass/function early in diabetes, a focus on relieving ER and inflammatory stress is also of interest to preserve beta cell health.
ER stress is a well known contributor to beta cell demise both in T1D and T2D (Laybutt et al., 2007 ; Marchetti et al., 2007 ; Marhfour et al., 2012 ; Tersey et al., 2012 ) and a target of interest in the prevention of beta cell loss in both diseases. Preclinical studies suggest that the use of chemical chaperones, including 4-phenylbutyric acid and tauroursodeoxycholic acid (TUDCA), to alleviate ER stress improves beta cell function and insulin sensitivity in mouse models of T2D (Cnop et al., 2017 ; Ozcan et al., 2006 ). Furthermore, TUDCA has been shown to preserve beta cell mass and reduce ER stress in mouse models of T1D (Engin et al., 2013 ). Interestingly, TUDCA has shown promise at improving insulin action in obese nondiabetic human subjects, yet beta cell function and insulin secretion were not assessed (Kars et al., 2010 ). A clinical trial regarding the use of TUDCA for humans with new-onset T1D is also ongoing (NCT02218619). However, a note of caution regarding use of ER chaperones is that they may prevent low level ER stress necessary to potentiate beta cell replication during states of increased insulin demand (Sharma et al., 2015 ), suggesting that the broad use of ER chaperone therapies should be carefully considered.
The blockade of inflammatory stress has long been an area of interest for treatments of both T1D and T2D (Donath et al., 2019 ; Eguchi and Nagai, 2017 ). Indeed, use of nonsteroidal anti-inflammatory drugs (NSAIDs), which block cyclooxygenase, have been observed to improve metabolic control in patients with diabetes since the turn of the 20th century (Williamson, 1901 ). Salicylates have been shown to improve insulin secretion and beta cell function in both obese human subjects and those with T2D (Fernandez-Real et al., 2008; Giugliano et al., 1985 ). However, another NSAID, salsalate, has not been shown to improve beta cell function while improving other metabolic outcomes (Kim et al., 2014 ; Penesova et al., 2015 ), possibly suggesting distinct mechanisms of action for anti-inflammatory compounds. The regular use of NSAIDs to enhance metabolic outcomes is also often limited to the tolerability of long-term use of these agents due to adverse effects. Recently, golilumab, a monoclonal antibody against the proinflammatory cytokine tumor necrosis factor alpha, was demonstrated to improve beta cell function in new-onset T1D, suggesting that targeting the underlying inflammatory milieu may have benefits to preserve beta cell mass and function in T1D (Quattrin et al., 2020). Taken together, both new and old approaches to target beta cell stressors still remain of long-term interest to improve beta cell viability and function in both T1D and T2D.
3. New Players to Induce Islet Immune Protection
Countless researchers have expended intense industry to determine T1D disease etiology and treatments focused on immunotherapy and tolerogenic methods. Multiple, highly comprehensive reviews are available describing these efforts (Goudy and Tisch, 2005 ; Rewers and Gottlieb, 2009 ; Stojanovic et al., 2017 ). Here we will focus on the protection of beta cells through programmed cell death protein-1 ligand (PD-L1) overexpression, major histocompatibility complex class I, A, B, C (HLA-A,B,C) mutated human embryonic stem cell–derived beta cells, and islet encapsulation methods.
Cancer immunotherapies that block immune checkpoints are beneficial for treating advanced stage cancers, yet induction of autoimmune diseases, including T1D, remains a potential side effect (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). A subset of these drugs target either the programmed cell death-1 protein on the surface of activated T lymphocytes or its receptor PD-L1 (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). PD-L1 expression was found in insulin-positive beta cells from T1D but not insulin-negative islets or nondiabetic islets, leading to the hypothesis that PD-L1 is upregulated in an attempt to drive immune cell attenuation (Osum et al., 2018 ; Colli et al., 2018 ). Adenoviral overexpression of PD-L1 specifically in beta cells rescued hyperglycemia in the NOD mouse model of T1D, but these animals eventually succumbed to diabetes by the study’s termination (El Khatib et al., 2015 ). A more promising report from Ben Nasr et al. ( 2017 ) demonstrated that pharmacologically or genetically induced overexpression of PD-L1 in hematopoietic stem and progenitor cells inhibited beta cell autoimmunity in the NOD mouse as well as in vitro using human hematopoietic stem and progenitor cells from patients with T1D.
As mentioned above, islet transplantation to treat T1D is limited by islet availability, cost, and the requirement for continuous immunosuppression. Islet cells generated by differentiating embryonic or induced pluripotent stem (iPS) cells could circumvent these limitations. Ideally, iPS-derived beta cells could be manipulated to eliminate the expression of polymorphic HLA-A,B,C molecules, which were found to be upregulated in T1D beta cells (Bottazzo et al., 1985 ; Richardson et al., 2016 ). These molecules allow peptide presentation to CD8+ T cells or cytotoxic T lymphocytes and may lead to beta cell removal. Interestingly, remaining insulin-positive cells in T1D donor pancreas are not HLA-A,B,C positive (Nejentsev et al., 2007; Rodriguez-Calvo et al., 2015 ). However, current differentiation protocols are still limited in their ability to produce fully glucose-responsive beta cells without transplantation into animal models to induce mature characteristics. Additionally, use of iPS-derived beta cells will still lead to concerns regarding DNA mutagenesis resulting from the methods used to obtain pluripotency or teratoma formation from cells that have escaped differentiation.
Encapsulation devices would protect islets or stem cells from immune cell infiltration while allowing for the proper exchange of nutrients and hormones. Macroencapsulation uses removable devices that would help assuage fears surrounding mutation or tumor formation; indeed, the first human trial using encapsulated hESC-derived beta cells will be completed in January 2021 (NCT02239354). Macroencapsulation of islets prior to transplantation using various alginate-based hydrogels has historically been impeded by a strong in vivo foreign body immune response (Desai and Shea, 2017 ; Doloff et al., 2017 ; Pueyo et al., 1993 ). More recently, chemically modified forms of alginate that avoid macrophage recognition and fibrous deposition have been successfully used in rodents and for up to 6 months in nonhuman primates (Vegas et al., 2016 ). Indeed, Bochenek et al. ( 2018 ) successfully transplanted alginate protected islets for 4 months without immunosuppression in the bursa omentalis of nonhuman primates demonstrating the feasibility for this approach to be extended to humans. It remains to be seen if these devices will be successful for long-term use, perhaps decades, in patients with diabetes.
III. Summary
Although existing drug therapies using classic oral antidiabetic drugs like sulfonylureas and metformin or injected insulin remain mainstays of diabetes treatment, newer drugs based on incretin hormone actions or SGLT2 inhibitors have increased the pharmacological armamentarium available to diabetologists ( Fig. 1 ). However, the explosion of progress in beta cell biology has identified potential avenues that can increase beta cell mass in sophisticated ways by employing stem cell differentiation or enhancement of beta cell proliferation. Taken together, there should be optimism that the increased incidence of both T1D and T2D is being matched by the creativity and hard work of the diabetes research community.
Abbreviations
| AMPK 5′ | AMP-activated protein kinase |
| CNS | central nervous system |
| DKA | diabetic ketoacidosis |
| DPP4 | dipeptidyl peptidase 4 |
| DYRK1A | dual specificity tyrosine phosphorylation-regulated kinase 1A |
| ER | endoplasmic reticulum |
| FDA | Food and Drug Administration |
| GIP | gastric inhibitory polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| GLP-1R | glucagon-like peptide-1 receptor |
| HLA-A,B,C | major histocompatibility complex class I, A, B, C |
| iPS | induced pluripotent stem |
| MEN1 | multiple endocrine neoplasia type 1 |
| NOD | nonobese diabetic |
| NSAID | nonsteroidal anti-inflammatory drug |
| PBA | phenylboronic acid |
| PD-L1 | programmed cell death protein-1 ligand |
| PPAR | peroxisome proliferator-activated receptor |
| SGLT2 | sodium glucose cotransporter 2 |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
| TUDCA | tauroursodeoxycholic acid |
| TXNIP | thioredoxin-interacting protein |
| TZD | thiazolidinedione. |
Authorship Contributions
Wrote and contributed to the writing of the manuscript: Satin, Soleimanpour, Walker
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01-DK46409] (to L.S.S.), [Grant R01-DK108921] (to S.A.S.), and [Grant P30-DK020572 pilot and feasibility grant] (to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF) [Grant CDA-2016-189] (to L.S.S. and S.A.S.), [Grant SRA-2018-539] (to S.A.S.), and [Grant COE-2019-861] (to S.A.S.), and the US Department of Veterans Affairs [Grant I01 BX004444] (to S.A.S.). The JDRF Career Development Award to S.A.S. is partly supported by the Danish Diabetes Academy and the Novo Nordisk Foundation.
https://doi.org/10.1124/pharmrev.120.000160
- Ackeifi C, Swartz E, Kumar K, Liu H, Chalada S, Karakose E, Scott DK, Garcia-Ocaña A, Sanchez R, DeVita RJ, et al. (2020) Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors . JCI Insight 5 :e132594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adachi T, Yasuda K, Okamoto Y, Shihara N, Oku A, Ueta K, Kitamura K, Saito A, Iwakura I, Yamada Y, et al. (2000) T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats . Metabolism 49 :990–995. [ PubMed ] [ Google Scholar ]
- Alexiadou K, Tan TM (2020) Gastrointestinal peptides as therapeutic targets to mitigate obesity and metabolic syndrome . Curr Diab Rep 20 :26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, et al. (2018) MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study . Lancet 391 :2607–2618. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell . Prog Biophys Mol Biol 54 :87–143. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1990) ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion . Biochem Soc Trans 18 :109–111. [ PubMed ] [ Google Scholar ]
- Baeyens L, Hindi S, Sorenson RL, German MS (2016) β-Cell adaptation in pregnancy . Diabetes Obes Metab 18 ( Suppl 1 ):63–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bailey CJ (1992) Biguanides and NIDDM . Diabetes Care 15 :755–772. [ PubMed ] [ Google Scholar ]
- Ben Nasr M, Tezza S, D’Addio F, Mameli C, Usuelli V, Maestroni A, Corradi D, Belletti S, Albarello L, Becchi G, et al. (2017) PD-L1 genetic overexpression or pharmacological restoration in hematopoietic stem and progenitor cells reverses autoimmune diabetes . Sci Transl Med 9 :eaam7543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, et al. (2019) Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study . Diabetologia 62 :665–675. [ PubMed ] [ Google Scholar ]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A (2014) Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map . Diabetes 63 :819–831. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al.; EXSCEL Study Group (2018) Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis . Lancet Diabetes Endocrinol 6 :105–113. [ PubMed ] [ Google Scholar ]
- Bliss M (1982) Banting’s, Best’s, and Collip’s accounts of the discovery of insulin . Bull Hist Med 56 :554–568. [ PubMed ] [ Google Scholar ]
- Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, et al. (2018) Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques . Nat Biomed Eng 2 :810–821. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion . Nat Med 21 :512–517. [ PubMed ] [ Google Scholar ]
- Bonora E, Cigolini M, Bosello O, Zancanaro C, Capretti L, Zavaroni I, Coscelli C, Butturini U (1984) Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects . Curr Med Res Opin 9 :47–51. [ PubMed ] [ Google Scholar ]
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis . N Engl J Med 313 :353–360. [ PubMed ] [ Google Scholar ]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence . Popul Health Metr 8 :29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment . Nat Med 13 :340–347. [ PubMed ] [ Google Scholar ]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M (2016) The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies . Diabetes Care 39 :198–205. [ PubMed ] [ Google Scholar ]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes . Diabetes Care 27 :2628–2635. [ PubMed ] [ Google Scholar ]
- Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, Jaffe JL, Coch RW, Haldeman JM, MacDonald PE, et al. (2019a) β Cell tone is defined by proglucagon peptides through cAMP signaling . JCI Insight 4 :e126742. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, D’Alessio DA, Campbell JE (2019b) Glucagon lowers glycemia when β-cells are active . JCI Insight 5 :e129954. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M (2017) Opposing effects of prostaglandin E 2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation . Mol Metab 6 :548–559. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chae H, Augustin R, Gatineau E, Mayoux E, Bensellam M, Antoine N, Khattab F, Lai BK, Brusa D, Stierstorfer B, et al. (2020) SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans . Mol Metab 42 :101071. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chamberlain CE, Scheel DW, McGlynn K, Kim H, Miyatsuka T, Wang J, Nguyen V, Zhao S, Mavropoulos A, Abraham AG, et al. (2014) Menin determines K-RAS proliferative outputs in endocrine cells . J Clin Invest 124 :4093–4101. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells . Nature 478 :349–355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, et al. (2015) Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells . Diabetes 64 :3784–3797. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Cha-Molstad H, Szabo A, Shalev A (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein . Am J Physiol Endocrinol Metab 296 :E1133–E1139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A (2008a) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes . FASEB J 22 :3581–3594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008b) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis . Diabetes 57 :938–944. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG (2015) Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates . Proc Natl Acad Sci USA 112 :2401–2406. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells . Mol Metab 6 :1024–1039. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans . J Clin Endocrinol Metab 88 :4696–4701. [ PubMed ] [ Google Scholar ]
- Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, et al. (2020) GDF15 mediates the effects of metformin on body weight and energy balance . Nature 578 :444–448. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colli ML, Hill JLE, Marroquí L, Chaffey J, Dos Santos RS, Leete P, Coomans de Brachène A, Paula FMM, Op de Beeck A, Castela A, et al. (2018) PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction . EBioMedicine 36 :367–375. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- RISE Consortium (2019) Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes . Diabetes Care 42 :1742–1751. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, et al. (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling . J Clin Invest 127 :3835–3844. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M, et al. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets . J Clin Invest 126 :1857–1870. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Walker JT, Shostak A, Bouchi Y, Poffenberger G, Hart NJ, Jacobson DA, Calcutt MW, Bottino R, Greiner DL, et al. (2020) Dapagliflozin does not directly affect human α or β cells . Endocrinology 161 :bqaa080. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR (2001) Oxyntomodulin inhibits food intake in the rat . Endocrinology 142 :4244–4250. [ PubMed ] [ Google Scholar ]
- Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR (2002) Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats . Am J Physiol Endocrinol Metab 283 :E1173–E1177. [ PubMed ] [ Google Scholar ]
- Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, et al.; ACT NOW Study (2013) Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates . Diabetes 62 :3920–3926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids . J Clin Invest 100 :2094–2098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Desai T, Shea LD (2017) Advances in islet encapsulation technologies . Nat Rev Drug Discov 16 :338–350. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Devaraj S, Venkatachalam A, Chen X (2016) Metformin and the gut microbiome in diabetes . Clin Chem 62 :1554–1555. [ PubMed ] [ Google Scholar ]
- Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, et al. (2017) Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates . Nat Mater 16 :671–680. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donath MY, Dinarello CA, Mandrup-Poulsen T (2019) Targeting innate immune mediators in type 1 and type 2 diabetes . Nat Rev Immunol 19 :734–746. [ PubMed ] [ Google Scholar ]
- Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1 . Cell Metab 27 :740–756. [ PubMed ] [ Google Scholar ]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line . Proc Natl Acad Sci USA 84 :3434–3438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats . Nat Med 21 :506–511. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eguchi K, Nagai R (2017) Islet inflammation in type 2 diabetes and physiology . J Clin Invest 127 :14–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El Khatib MM, Sakuma T, Tonne JM, Mohamed MS, Holditch SJ, Lu B, Kudva YC, Ikeda Y (2015) β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection . Gene Ther 22 :430–438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, et al. (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial . Lancet 389 :369–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons . J Mol Neurosci 34 :77–87. [ PubMed ] [ Google Scholar ]
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I . J Biol Chem 275 :223–228. [ PubMed ] [ Google Scholar ]
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y (1964) Plasma Insulin Response to Oral and Intravenous Glucose Administration . J Clin Endocrinol Metab 24 :1076–1082. [ PubMed ] [ Google Scholar ]
- Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med (2013) 5 :214er11] . Sci Transl Med 5 :211ra156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients . BMJ 330 :1304–1305. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, et al. (2009) Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure . Mol Cell Biol 29 :2053–2067. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fernández-Real JM, López-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, Casamitjana R, Gomis R, Arnaiz E, Pérez I, et al. (2008) Salicylates increase insulin secretion in healthy obese subjects . J Clin Endocrinol Metab 93 :2523–2530. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies . Cell Metab 20 :953–966. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus . Nat Rev Endocrinol 15 :569–589. [ PubMed ] [ Google Scholar ]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state . J Clin Invest 120 :2355–2369. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JPBastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RHDiMarchi RD (2017) The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes . Cell Metab 26 :343–352.e2. [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA (2020) Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens . Diabetes Obes Metab 22 :938–946. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, et al. (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial . Lancet 392 :2180–2193. [ PubMed ] [ Google Scholar ]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin . Nat Med 19 :1649–1654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al.; REWIND Investigators (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial . Lancet 394 :121–130. [ PubMed ] [ Google Scholar ]
- Giugliano D, Ceriello A, Saccomanno F, Quatraro A, Paolisso G, D’Onofrio F (1985) Effects of salicylate, tolbutamide, and prostaglandin E2 on insulin responses to glucose in noninsulin-dependent diabetes mellitus . J Clin Endocrinol Metab 61 :160–166. [ PubMed ] [ Google Scholar ]
- Goudy KS, Tisch R (2005) Immunotherapy for the prevention and treatment of type 1 diabetes . Int Rev Immunol 24 :307–326. [ PubMed ] [ Google Scholar ]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ (2012) Formation of a human β-cell population within pancreatic islets is set early in life . J Clin Endocrinol Metab 97 :3197–3206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P (2012) Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors . Diabetes Obes Metab 14 :83–90. [ PubMed ] [ Google Scholar ]
- Harlan DM (2016) Islet transplantation for hypoglycemia unawareness/severe hypoglycemia: caveat emptor . Diabetes Care 39 :1072–1074. [ PubMed ] [ Google Scholar ]
- Harrower AD (1991) Efficacy of gliclazide in comparison with other sulphonylureas in the treatment of NIDDM . Diabetes Res Clin Pract 14 ( Suppl 2 ):S65–S67. [ PubMed ] [ Google Scholar ]
- He L, Wondisford FE (2015) Metformin action: concentrations matter . Cell Metab 21 :159–162. [ PubMed ] [ Google Scholar ]
- Hedrington MS, Davis SN (2019) Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes . Expert Opin Pharmacother 20 :2229–2235. [ PubMed ] [ Google Scholar ]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.; Clinical Islet Transplantation Consortium (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia . Diabetes Care 39 :1230–1240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, et al. (2005) Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes . JAMA 293 :830–835. [ PubMed ] [ Google Scholar ]
- Hernandez AFGreen JBJanmohamed SD’Agostino RB Sr , Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MCet al.; Harmony Outcomes committees and investigators (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial . Lancet 392 :1519–1529. [ PubMed ] [ Google Scholar ]
- Hiatt WR, Kaul S, Smith RJ (2013) The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience . N Engl J Med 369 :1285–1287. [ PubMed ] [ Google Scholar ]
- Hirshberg B, Preston EH, Xu H, Tal MG, Neeman Z, Bunnell D, Soleimanpour S, Hale DA, Kirk AD, Harlan DM (2003) Rabbit antithymocyte globulin induction and sirolimus monotherapy supports prolonged islet allograft function in a nonhuman primate islet transplantation model . Transplantation 76 :55–60. [ PubMed ] [ Google Scholar ]
- Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, et al. (2019) Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response . Cell Stem Cell 24 :299–308.e6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al.; PIONEER 6 Investigators (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 381 :841–851. [ PubMed ] [ Google Scholar ]
- Imamura M, Nakanishi K, Suzuki T, Ikegai K, Shiraki R, Ogiyama T, Murakami T, Kurosaki E, Noda A, Kobayashi Y, et al. (2012) Discovery of Ipragliflozin (ASP1941): a novel C-glucoside with benzothiophene structure as a potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus . Bioorg Med Chem 20 :3263–3279. [ PubMed ] [ Google Scholar ]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al.; ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy . N Engl J Med 355 :2427–2443. [ PubMed ] [ Google Scholar ]
- Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR; ADOPT Study Group (2011) Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT . Diabetes 60 :1552–1560. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, et al. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women . Diabetes 59 :1899–1905. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF (1973) Transplantation of isolated pancreatic islets into the portal vein of diabetic rats . Nature 244 :447. [ PubMed ] [ Google Scholar ]
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH (2002) Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells . Diabetes 51 :676–685. [ PubMed ] [ Google Scholar ]
- Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH (2000) Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter . Diabetes 49 :1517–1524. [ PubMed ] [ Google Scholar ]
- Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G (2014) Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study . Diabetes Care 37 :1944–1950. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koffert JP, Mikkola K, Virtanen KA, Andersson AD, Faxius L, Hällsten K, Heglind M, Guiducci L, Pham T, Silvola JMU, et al. (2017) Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial . Diabetes Res Clin Pract 131 :208–216. [ PubMed ] [ Google Scholar ]
- Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Bäckhed F (2020) Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation . Cell Metab 32 :643–653.e4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7-36: a physiological incretin in man . Lancet 2 :1300–1304. [ PubMed ] [ Google Scholar ]
- Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Ørskov C, Gribble FM, et al. (2019) No direct effect of SGLT2 activity on glucagon secretion . Diabetologia 62 :1011–1023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF (2012) Human β-cell proliferation and intracellular signaling: driving in the dark without a road map . Diabetes 61 :2205–2213. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kurosaki E, Ogasawara H (2013) Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data . Pharmacol Ther 139 :51–59. [ PubMed ] [ Google Scholar ]
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use . Diabetes 49 :999–1005. [ PubMed ] [ Google Scholar ]
- Lambeir AM, Scharpé S, De Meester I (2008) DPP4 inhibitors for diabetes--what next? Biochem Pharmacol 76 :1637–1643. [ PubMed ] [ Google Scholar ]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes . Diabetologia 50 :752–763. [ PubMed ] [ Google Scholar ]
- Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications . Curr Diab Rep 19 :151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, et al. (2015) Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes . JAMA 314 :265–277. [ PubMed ] [ Google Scholar ]
- Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, et al. (2016) Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies . BMJ 352 :i610. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Link JT (2003) Pharmacological regulation of hepatic glucose production . Curr Opin Investig Drugs 4 :421–429. [ PubMed ] [ Google Scholar ]
- Luippold G, Klein T, Mark M, Grempler R (2012) Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus . Diabetes Obes Metab 14 :601–607. [ PubMed ] [ Google Scholar ]
- Maganti AV, Tersey SA, Syed F, Nelson JB, Colvin SC, Maier B, Mirmira RG (2016) Peroxisome proliferator-activated receptor-γ activation augments the β-cell unfolded protein response and rescues early glycemic deterioration and β cell death in non-obese diabetic mice . J Biol Chem 291 :22524–22533. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Makrilakis K (2019) The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect . Int J Environ Res Public Health 16 :2720. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M (2007) The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients . Diabetologia 50 :2486–2494. [ PubMed ] [ Google Scholar ]
- Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, Eizirik DL (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes . Diabetologia 55 :2417–2420. [ PubMed ] [ Google Scholar ]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al.; SUSTAIN-6 Investigators (2016a) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 375 :1834–1844. [ PubMed ] [ Google Scholar ]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al.; LEADER Steering Committee; LEADER Trial Investigators (2016b) Liraglutide and cardiovascular outcomes in type 2 diabetes . N Engl J Med 375 :311–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Massollo M, Marini C, Brignone M, Emionite L, Salani B, Riondato M, Capitanio S, Fiz F, Democrito A, Amaro A, et al. (2013) Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice . J Nucl Med 54 :259–266. [ PubMed ] [ Google Scholar ]
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract . Diabetologia 59 :426–435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McIntyre N, Holdsworth CD, Turner DS (1964) New interpretation of oral glucose tolerance . Lancet 2 :20–21. [ PubMed ] [ Google Scholar ]
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al.; DAPA-HF Trial Committees and Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction . N Engl J Med 381 :1995–2008. [ PubMed ] [ Google Scholar ]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans . Diabetes 57 :1584–1594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, et al. (2008) Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes . J Med Chem 51 :1145–1149. [ PubMed ] [ Google Scholar ]
- Menting JG, Whittaker J, Margetts MB, Whittaker LJ, Kong GK, Smith BJ, Watson CJ, Záková L, Kletvíková E, Jiráček J, et al. (2013) How insulin engages its primary binding site on the insulin receptor . Nature 493 :241–245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP . Nature 494 :256–260. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Minkowski O (1892) Weitere Mitteilungen über den Diabetes mellitus nach Extirpation des Pankreas . Berliner Klinische Wochenschrift 29 :90–93. [ Google Scholar ]
- Misbin RI (2004) The phantom of lactic acidosis due to metformin in patients with diabetes . Diabetes Care 27 :1791–1793. [ PubMed ] [ Google Scholar ]
- Mudaliar S, Armstrong DA, Mavian AA, O’Connor-Semmes R, Mydlow PK, Ye J, Hussey EK, Nunez DJ, Henry RR, Dobbins RL (2012) Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes . Diabetes Care 35 :2198–2200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL (2011) Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell . Endocrinology 152 :4610–4619. [ PubMed ] [ Google Scholar ]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. (2019) Glucagon-like peptide 1 (GLP-1) . Mol Metab 30 :72–130. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Munir KM, Lamos EM (2017) Diabetes type 2 management: what are the differences between DPP-4 inhibitors and how do you choose? Expert Opin Pharmacother 18 :839–841. [ PubMed ] [ Google Scholar ]
- Naftanel MA, Harlan DM (2004) Pancreatic islet transplantation . PLoS Med 1 :e58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986a) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes . Diabetologia 29 :46–52. [ PubMed ] [ Google Scholar ]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W (1986b) Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses . J Clin Endocrinol Metab 63 :492–498. [ PubMed ] [ Google Scholar ]
- Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, et al.; Wellcome Trust Case Control Consortium (2007) Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A . Nature 450 :887–892. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nichols CG (2006) KATP channels as molecular sensors of cellular metabolism . Nature 440 :470–476. [ PubMed ] [ Google Scholar ]
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, et al. (2010) Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus . J Med Chem 53 :6355–6360. [ PubMed ] [ Google Scholar ]
- Ohnishi ST, Endo M, editors. (1981) The Mechanism of Gated Calcium Transport Across Biological Membranes , Academic Press, New York. [ Google Scholar ]
- Osipovich AB, Stancill JS, Cartailler JP, Dudek KD, Magnuson MA (2020) Excitotoxicity and overnutrition additively impair metabolic function and identity of pancreatic β-cells . Diabetes 69 :1476–1491. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA, et al. (2018) Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes . Sci Rep 8 :8295. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain . Biochem J 348 :607–614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ozanne SE, Guest PC, Hutton JC, Hales CN (1995) Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells . Diabetologia 38 :277–282. [ PubMed ] [ Google Scholar ]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes . Science 313 :1137–1140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paty BW, Harmon JS, Marsh CL, Robertson RP (2002) Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets . Transplantation 73 :353–357. [ PubMed ] [ Google Scholar ]
- Penesova A, Koska J, Ortega E, Bunt JC, Bogardus C, de Courten B (2015) Salsalate has no effect on insulin secretion but decreases insulin clearance: a randomized, placebo-controlled trial in subjects without diabetes . Diabetes Obes Metab 17 :608–612. [ PubMed ] [ Google Scholar ]
- Perdigoto AL, Quandt Z, Anderson M, Herold KC (2019) Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome . Lancet Diabetes Endocrinol 7 :421–423. [ PubMed ] [ Google Scholar ]
- Perley MJ, Kipnis DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects . J Clin Invest 46 :1954–1962. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer . Nat Rev Endocrinol 10 :143–156. [ PubMed ] [ Google Scholar ]
- Preiss D, Dawed A, Welsh P, Heggie A, Jones AG, Dekker J, Koivula R, Hansen TH, Stewart C, Holman RR, et al.; DIRECT consortium group (2017) Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes . Diabetes Obes Metab 19 :356–363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes . J Clin Invest 116 :1802–1812. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pueyo ME, Darquy S, Capron F, Reach G (1993) In vitro activation of human macrophages by alginate-polylysine microcapsules . J Biomater Sci Polym Ed 5 :197–203. [ PubMed ] [ Google Scholar ]
- Pybus F (1924) Notes on suprarenal and pancreatic grafting . Lancet 204 :550–551. [ Google Scholar ]
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, et al.; T1GER Study Investigators (2020) Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes . N Engl J Med 383 :2007–2017. [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Aïello V, Herault Y, Janel N, Delabar JM, Polak M, Scharfmann R (2014a) Dyrk1A induces pancreatic β cell mass expansion and improves glucose tolerance . Cell Cycle 13 :2221–2229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R (2014b) Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass . Diabetologia 57 :960–969. [ PubMed ] [ Google Scholar ]
- Rege NK, Phillips NFB, Weiss MA (2017) Development of glucose-responsive ‘smart’ insulin systems . Curr Opin Endocrinol Diabetes Obes 24 :267–278. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin . Diabetologia 60 :1577–1585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rewers M, Gottlieb P (2009) Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future . Diabetes Care 32 :1769–1782. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, et al. (2016) Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes . Diabetologia 59 :2448–2458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, et al. (2013) Improvement in β-cell secretory capacity after human islet transplantation according to the c7 protocol . Diabetes 62 :2890–2897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Robertson RP (2019) Pancreatic islet transplantation in humans: recent progress and future directions . Endocr Rev 40 :631–668. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG (2015) Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase . J Histochem Cytochem 63 :626–636. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rojas LB, Gomes MB (2013) Metformin: an old but still the best treatment for type 2 diabetes . Diabetol Metab Syndr 5 :6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. (2003) Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis . Mol Cell Biol 23 :7222–7229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM; Repaglinide Versus Nateglinide Comparison Study Group (2004) Repaglinide versus nateglinide monotherapy: a randomized, multicenter study . Diabetes Care 27 :1265–1270. [ PubMed ] [ Google Scholar ]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients . Clin J Am Soc Nephrol 6 :1198–1206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, Coddeville A, Quenon A, Daoudi M, Hubert T, et al. (2019) The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release . Cell Rep 28 :1447–1454.e4. [ PubMed ] [ Google Scholar ]
- Saponaro C, Mühlemann M, Acosta-Montalvo A, Piron A, Gmyr V, Delalleau N, Moerman E, Thévenet J, Pasquetti G, Coddeville A, et al. (2020) Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets . Diabetes 69 :902–914. [ PubMed ] [ Google Scholar ]
- Satin LS, Tavalin SJ, Kinard TA, Teague J (1995) Contribution of L- and non-L-type calcium channels to voltage-gated calcium current and glucose-dependent insulin secretion in HIT-T15 cells . Endocrinology 136 :4589–4601. [ PubMed ] [ Google Scholar ]
- Schwartz AV, Chen H, Ambrosius WT, Sood A, Josse RG, Bonds DE, Schnall AM, Vittinghoff E, Bauer DC, Banerji MA, et al. (2015) Effects of TZD use and discontinuation on fracture rates in ACCORD Bone Study . J Clin Endocrinol Metab 100 :4059–4066. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM (2002) Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway . Endocrinology 143 :3695–3698. [ PubMed ] [ Google Scholar ]
- Shankar SS, Shankar RR, Mixson LA, Miller DL, Pramanik B, O’Dowd AK, Williams DM, Frederick CB, Beals CR, Stoch SA, et al. (2018) Native oxyntomodulin has significant glucoregulatory effects independent of weight loss in obese humans with and without type 2 diabetes . Diabetes 67 :1105–1112. [ PubMed ] [ Google Scholar ]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen . N Engl J Med 343 :230–238. [ PubMed ] [ Google Scholar ]
- Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC (2015) Insulin demand regulates β cell number via the unfolded protein response . J Clin Invest 125 :3831–3846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sims EK, Mirmira RG, Evans-Molina C (2020) The role of beta-cell dysfunction in early type 1 diabetes . Curr Opin Endocrinol Diabetes Obes 27 :215–224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang B, Everett LJ, et al. (2015) Genetic variation determines PPARγ function and anti-diabetic drug response in vivo . Cell 162 :33–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, et al. (2010) Calcineurin signaling regulates human islet beta-cell survival . J Biol Chem 285 :40050–40059. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Hirshberg B, Bunnell DJ, Sumner AE, Ader M, Remaley AT, Rother KI, Rickels MR, Harlan DM (2012) Metabolic function of a suboptimal transplanted islet mass in nonhuman primates on rapamycin monotherapy . Cell Transplant 21 :1297–1304. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Stoffers DA (2013) The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 24 :324–331. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. (2018) Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors . Diabetes 67 :1471–1480. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stancill JS, Cartailler JP, Clayton HW, O’Connor JT, Dickerson MT, Dadi PK, Osipovich AB, Jacobson DA, Magnuson MA (2017) Chronic β-cell depolarization impairs β-cell identity by disrupting a network of Ca 2+ -regulated genes . Diabetes 66 :2175–2187. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stewart AF, Hussain MA, García-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN (2015) Human β-cell proliferation and intracellular signaling: part 3 . Diabetes 64 :1872–1885. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stojanovic I, Dimitrijevic M, Vives-Pi M, Mansilla MJ, Pujol-Autonell I, Rodríguez-Fernandez S, Palova-Jelínkova L, Funda DP, Gruden-Movsesijan A, Sofronic-Milosavljevic L, et al. (2017) Cell-based tolerogenic therapy, experience from animal models of multiple sclerosis, type 1 diabetes and rheumatoid arthritis . Curr Pharm Des 23 :2623–2643. [ PubMed ] [ Google Scholar ]
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC (2010) An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells . J Clin Invest 120 :2575–2589. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, et al. (2019) SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels . Mol Metab 19 :1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin . Nat Med 24 :1919–1929. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sutherland DE, Matas AJ, Najarian JS (1978) Pancreatic islet cell transplantation . Surg Clin North Am 58 :365–382. [ PubMed ] [ Google Scholar ]
- Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model . Diabetes 61 :818–827. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thielen LA, Chen J, Jing G, Moukha-Chafiq O, Xu G, Jo S, Grayson TB, Lu B, Li P, Augelli-Szafran CE, et al. (2020) Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action . Cell Metab 32 :353–365.e8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, et al. (2019) A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials . Diabetes Obes Metab 21 :120–128. [ PubMed ] [ Google Scholar ]
- Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus . Annu Rev Med 66 :255–270. [ PubMed ] [ Google Scholar ]
- Vasseur M, Debuyser A, Joffre M (1987) Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine . Fundam Clin Pharmacol 1 :95–113. [ PubMed ] [ Google Scholar ]
- Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, et al. (2016) Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates . Nat Biotechnol 34 :345–352. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, Asterholm IW, Benrick A, Briant LJB, Chibalina MV, et al. (2019) Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion . Nat Commun 10 :139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wallner K, Shapiro AM, Senior PA, McCabe C (2016) Cost effectiveness and value of information analyses of islet cell transplantation in the management of ‘unstable’ type 1 diabetes mellitus . BMC Endocr Disord 16 :17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang H, Bender A, Wang P, Karakose E, Inabnet WB, Libutti SK, Arnold A, Lambertini L, Stang M, Chen H, et al. (2017) Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas . Nat Commun 8 :767. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK . Cell Rep 29 :1511–1523.e5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Westerman J, Wirtz KW, Berkhout T, van Deenen LL, Radhakrishnan R, Khorana HG (1983) Identification of the lipid-binding site of phosphatidylcholine-transfer protein with phosphatidylcholine analogs containing photoactivable carbene precursors . Eur J Biochem 132 :441–449. [ PubMed ] [ Google Scholar ]
- World Health Organization (2020) World Health Organization Diabetes Fact Sheet . [ Google Scholar ]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist . JCI Insight 5 :e140532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams P (1894) Notes on diabetes treated with extract and by grafts of sheep’s pancreas . BMJ 2 :1303–1304. [ Google Scholar ]
- Williamson RT (1901) On the treatment of glycosuria and diabetes mellitus with sodium salicylate . BMJ 1 :760–762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Witters LA (2001) The blooming of the French lilac . J Clin Invest 108 :1105–1107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al.; DECLARE–TIMI 58 Investigators (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes . N Engl J Med 380 :347–357. [ PubMed ] [ Google Scholar ]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug . Nat Med 23 :850–858. [ PubMed ] [ Google Scholar ]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. (2005) Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial . Diabetes 54 :2390–2395. [ PubMed ] [ Google Scholar ]
- Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers . Diabetes 61 :848–856. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang JF, Gong X, Bakh NA, Carr K, Phillips NFB, Ismail-Beigi F, Weiss MA, Strano MS (2020) Connecting rodent and human pharmacokinetic models for the design and translation of glucose-responsive insulin . Diabetes 69 :1815–1826. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu O, Azoulay L, Yin H, Filion KB, Suissa S (2018) Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia . Am J Med 131 :317.e11–317.e22. [ PubMed ] [ Google Scholar ]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action . J Clin Invest 108 :1167–1174. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al.; EMPA-REG OUTCOME Investigators (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes . N Engl J Med 373 :2117–2128. [ PubMed ] [ Google Scholar ]

COMMENTS
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. ... Research Highlights 02 Aug 2024 ...
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the ...
2. Type 1 Diabetes Mellitus. Type 1 diabetes mellitus (T1DM) incidence has been increasing by 2-5% worldwide with significant heterogeneity in this diagnosis by regions or continents [5,6].Clinical care has significantly improved, raising quality of life and clinical outcomes for these patients, but more must be done to find a cure.
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
Previously, type 1 diabetes was called insulin-dependent diabetes and it could happen at any age but is most common in children and young people. 3. People with type 1 diabetes are not able to produce enough insulin. This type constitutes about 5%-10% of all cases of diabetes.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease affecting 8.75 million people worldwide, of whom ~1.52 million are <20 years old 1.According to the US Centers for Disease Control and ...
A focus of type 1 diabetes research is preventing the autoimmune destruction of the pancreatic β cell. 166 Primary strategies to prevent the onset of autoimmunity in infants at high risk for developing type 1 diabetes by modifying diet or introducing supplements in early life have not succeeded. 167-169 Antigen-specific immunotherapy has ...
Hypoglycaemia is a common occurrence in people with type 1 diabetes mellitus, and can have serious consequences. This Review defines hypoglycaemia in type 1 diabetes mellitus, and also outlines ...
While several valid and reliable measures of diabetes-specific QoL exist, there is no consensus on which to use and in what setting. Furthermore, there is limited evidence of their acceptability to people with diabetes. Our aim was to explore perceptions of adults with type 1 diabetes (T1D) toward five diabetes-specific QoL measures.
Type-1 diabetes is a multifactorial disease characterized by genetic and environmental factors that contribute to its development and progression. Despite progress in the management of type-1 diabetes, the final goal of curing the disease is yet to be achieved. To establish effective methods for the prevention, intervention, and cure of the disease, the molecular mechanisms and pathways ...
Join the movement to end T1D. Champion federal funding for T1D research. Inform health and regulatory policy. Improve lives. Breakthrough T1D is the leading global type 1 diabetes (T1D) research and advocacy organization. As we drive toward curing type 1 diabetes, we help make everyday life better for the people who face it.
The Clinical Research in Type 1 Diabetes program includes studies across the lifespan that address the etiology, pathogenesis, prevention and treatment (medical- and self-management) of type 1 diabetes in youth and adults. The program also supports research on hypoglycemia in T1D, including clinical studies and basic research using healthy ...
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
Diabetes-specific quality of life (QoL) refers to an individual's perception of the impact of diabetes on their QoL, or how diabetes affects aspects of life important to them [1, 2].Several diabetes-specific QoL assessment tools exist [2,3,4,5], with no consensus on which to use and in what setting.Likely relatedly, there has also been a lack of systematic QoL assessment (diabetes-specific ...
Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (T1D).
New technologies in type 1 diabetes. Intensive insulin therapy for the management of type 1 diabetes (T1D) was established as the standard of care based on the results of the Diabetes Control and Complication Trial (DCCT), which conclusively demonstrated the benefits of tight glycemic control. 1 However, those who received intensive insulin management were at increased risk for severe ...
Review Article: Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead.Image Credit: Andrii Yalanskyi / Shutterstock. In a recent study published ...
DRI clinical trials are already dramatically improving the lives of some people with type 1 diabetes who are now living insulin-free. The Institute's scientists are addressing the major research challenges that stand in the way of a biological cure. But continuing this research is only possible with your support.
Type 1 Research Highlights. While the Association's priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes. Read more about the critical research made possible by the ...
Automated insulin delivery improves glycaemic control and pregnancy outcomes in pregnancy complicated by maternal type 1 diabetes mellitus (T1DM) compared to standard of care 2.. Once-weekly basal ...
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. In this condition, the pancreas makes little or no insulin. ... Even after a lot of research, type 1 diabetes has no cure. Treatment is directed toward managing the amount of sugar in the blood using insulin, diet and lifestyle to prevent ...
The American Diabetes Association is currently a partner providing support for the following clinical studies and initiatives: TrialNet. Type 1 Diabetes TrialNet is an international network of researchers who are exploring ways to prevent, delay and reverse the progression of type 1 diabetes. GRADE. GRADE is a comparative effectiveness study ...
Hundreds of people with type 1 diabetes, their families and healthcare professionals have chosen their most pressing research priorities for type 1 diabetes. The top ten priorities will help to guide future type 1 diabetes research in the UK and Ireland to make sure it has the greatest possible benefit for people with the condition.
Type 1 diabetes risk after age 10 differs between boys and girls; A total of 19 adults with type 1 diabetes took part in the study. The research team looked at each participant's glycaemic and cardiovascular responses following interval exercise sessions and continuous exercise sessions.
The SEARCH for Diabetes in Youth study in the United States found a 1.4% per year increase in T1D incidence from 2002 to 2012, with an unexpected increase in Hispanic youths (14). This increase in the United States is similar to the gradual worldwide annual increase in T1D incidence over the past 30 years.
This groundbreaking research shows promise as the first disease-modifying treatment of its kind for type 1 diabetes that can be delivered as a tablet. "It is tremendously exciting for us to be the first group anywhere in the world to test the efficacy of baricitinib as a potential type 1 diabetes treatment," Professor Kay says.
Although type 2 diabetes is the most common type of diabetes in older adults, type 1 diabetes is becoming more common as people with the disease are living longer because of improved insulin delivery, technology, and care. We expect the number of older adults living with type 1 or type 2 diabetes to increase markedly in the coming decades.
Everything You Need To Know About Type 1 Diabetes; Study: Talking At This Speed Could Signal Dementia ... However, initial research conducted on semaglutide (the active ingredient in Ozempic) and ...
Both type 1 and type 2 diabetes mellitus are advancing at exponential rates, placing significant burdens on health care networks worldwide. Although traditional pharmacologic therapies such as insulin and oral antidiabetic stalwarts like metformin and the sulfonylureas continue to be used, newer drugs are now on the market targeting novel blood glucose-lowering pathways.