- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About Journal of Molecular Cell Biology
- Society affiliations
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Obesity: causes, consequences, treatments, and challenges.
- Article contents
- Figures & tables
- Supplementary Data
Obesity: causes, consequences, treatments, and challenges, Journal of Molecular Cell Biology , Volume 13, Issue 7, July 2021, Pages 463–465, https://doi.org/10.1093/jmcb/mjab056
- Permissions Icon Permissions
Obesity has become a global epidemic and is one of today’s most public health problems worldwide. Obesity poses a major risk for a variety of serious diseases including diabetes mellitus, non-alcoholic liver disease (NAFLD), cardiovascular disease, hypertension and stroke, and certain forms of cancer ( Bluher, 2019 ).
Obesity is mainly caused by imbalanced energy intake and expenditure due to a sedentary lifestyle coupled with overnutrition. Excess nutrients are stored in adipose tissue (AT) in the form of triglycerides, which will be utilized as nutrients by other tissues through lipolysis under nutrient deficit conditions. There are two major types of AT, white AT (WAT) and brown AT, the latter is a specialized form of fat depot that participates in non-shivering thermogenesis through lipid oxidation-mediated heat generation. While WAT has been historically considered merely an energy reservoir, this fat depot is now well known to function as an endocrine organ that produces and secretes various hormones, cytokines, and metabolites (termed as adipokines) to control systemic energy balance. Studies over the past decade also show that WAT, especially subcutaneous WAT, could undergo ‘beiging’ remodeling in response to environmental or hormonal perturbation. In the first paper of this special issue, Cheong and Xu (2021) systematically review the recent progress on the factors, pathways, and mechanisms that regulate the intercellular and inter-organ crosstalks in the beiging of WAT. A critical but still not fully addressed issue in the adipose research field is the origin of the beige cells. Although beige adipocytes are known to have distinct cellular origins from brown and while adipocytes, it remains unclear on whether the cells are from pre-existing mature white adipocytes through a transdifferentiation process or from de novo differentiation of precursor cells. AT is a heterogeneous tissue composed of not only adipocytes but also nonadipocyte cell populations, including fibroblasts, as well as endothelial, blood, stromal, and adipocyte precursor cells ( Ruan, 2020 ). The authors examined evidence to show that heterogeneity contributes to different browning capacities among fat depots and even within the same depot. The local microenvironment in WAT, which is dynamically and coordinately controlled by inputs from the heterogeneous cell types, plays a critical role in the beige adipogenesis process. The authors also examined key regulators of the AT microenvironment, including vascularization, the sympathetic nerve system, immune cells, peptide hormones, exosomes, and gut microbiota-derived metabolites. Given that increasing beige fat function enhances energy expenditure and consequently reduces body weight gain, identification and characterization of novel regulators and understanding their mechanisms of action in the beiging process has a therapeutic potential to combat obesity and its associated diseases. However, as noticed by the authors, most of the current pre-clinical research on ‘beiging’ are done in rodent models, which may not represent the exact phenomenon in humans ( Cheong and Xu, 2021 ). Thus, further investigations will be needed to translate the findings from bench to clinic.
While both social–environmental factors and genetic preposition have been recognized to play important roles in obesity epidemic, Gao et al. (2021) present evidence showing that epigenetic changes may be a key factor to explain interindividual differences in obesity. The authors examined data on the function of DNA methylation in regulating the expression of key genes involved in metabolism. They also summarize the roles of histone modifications as well as various RNAs such as microRNAs, long noncoding RNAs, and circular RNAs in regulating metabolic gene expression in metabolic organs in response to environmental cues. Lastly, the authors discuss the effect of lifestyle modification and therapeutic agents on epigenetic regulation of energy homeostasis. Understanding the mechanisms by which lifestyles such as diet and exercise modulate the expression and function of epigenetic factors in metabolism should be essential for developing novel strategies for the prevention and treatment of obesity and its associated metabolic diseases.
A major consequence of obesity is type 2 diabetes, a chronic disease that occurs when body cannot use and produce insulin effectively. Diabetes profoundly and adversely affects the vasculature, leading to various cardiovascular-related diseases such as atherosclerosis, arteriosclerotic, and microvascular diseases, which have been recognized as the most common causes of death in people with diabetes ( Cho et al., 2018 ). Love et al. (2021) systematically review the roles and regulation of endothelial insulin resistance in diabetes complications, focusing mainly on vascular dysfunction. The authors review the vasoprotective functions and the mechanisms of action of endothelial insulin and insulin-like growth factor 1 signaling pathways. They also examined the contribution and impart of endothelial insulin resistance to diabetes complications from both biochemical and physiological perspectives and evaluated the beneficial roles of many of the medications currently used for T2D treatment in vascular management, including metformin, thiazolidinediones, glucagon-like receptor agonists, dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter inhibitors, as well as exercise. The authors present evidence to suggest that sex differences and racial/ethnic disparities contribute significantly to vascular dysfunction in the setting of diabetes. Lastly, the authors raise a number of very important questions with regard to the role and connection of endothelial insulin resistance to metabolic dysfunction in other major metabolic organs/tissues and suggest several insightful directions in this area for future investigation.
Following on from the theme of obesity-induced metabolic dysfunction, Xia et al. (2021) review the latest progresses on the role of membrane-type I matrix metalloproteinase (MT1-MMP), a zinc-dependent endopeptidase that proteolytically cleaves extracellular matrix components and non-matrix proteins, in lipid metabolism. The authors examined data on the transcriptional and post-translational modification regulation of MT1-MMP gene expression and function. They also present evidence showing that the functions of MT1-MMP in lipid metabolism are cell specific as it may either promote or suppress inflammation and atherosclerosis depending on its presence in distinct cells. MT1-MMP appears to exert a complex role in obesity for that the molecule delays the progression of early obesity but exacerbates obesity at the advanced stage. Because inhibition of MT1-MMP can potentially lower the circulating low-density lipoprotein cholesterol levels and reduce the risk of cancer metastasis and atherosclerosis, the protein has been viewed as a very promising therapeutic target. However, challenges remain in developing MT1-MMP-based therapies due to the tissue-specific roles of MT1-MMP and the lack of specific inhibitors for this molecule. Further investigations are needed to address these questions and to develop MT1-MMP-based therapeutic interventions.
Lastly, Huang et al. (2021) present new findings on a critical role of puromycin-sensitive aminopeptidase (PSA), an integral non-transmembrane enzyme that catalyzes the cleavage of amino acids near the N-terminus of polypeptides, in NAFLD. NAFLD, ranging from simple nonalcoholic fatty liver to the more aggressive subtype nonalcoholic steatohepatitis, has now become the leading chronic liver disease worldwide ( Loomba et al., 2021 ). At present, no effective drugs are available for NAFLD management in the clinic mainly due to the lack of a complete understanding of the mechanisms underlying the disease progress, reinforcing the urgent need to identify and validate novel targets and to elucidate their mechanisms of action in NAFLD development and pathogenesis. Huang et al. (2021) found that PSA expression levels were greatly reduced in the livers of obese mouse models and that the decreased PSA expression correlated with the progression of NAFLD in humans. They also found that PSA levels were negatively correlated with triglyceride accumulation in cultured hepatocytes and in the liver of ob/ob mice. Moreover, PSA suppresses steatosis by promoting lipogenesis and attenuating fatty acid β-oxidation in hepatocytes and protects oxidative stress and lipid overload in the liver by activating the nuclear factor erythroid 2-related factor 2, the master regulator of antioxidant response. These studies identify PSA as a pivotal regulator of hepatic lipid metabolism and suggest that PSA may be a potential biomarker and therapeutic target for treating NAFLD.
In summary, papers in this issue review our current knowledge on the causes, consequences, and interventions of obesity and its associated diseases such as type 2 diabetes, NAFLD, and cardiovascular disease ( Cheong and Xu, 2021 ; Gao et al., 2021 ; Love et al., 2021 ). Potential targets for the treatment of dyslipidemia and NAFLD are also discussed, as exemplified by MT1-MMP and PSA ( Huang et al., 2021 ; Xia et al., 2021 ). It is noted that despite enormous effect, few pharmacological interventions are currently available in the clinic to effectively treat obesity. In addition, while enhancing energy expenditure by browning/beiging of WAT has been demonstrated as a promising alternative approach to alleviate obesity in rodent models, it remains to be determined on whether such WAT reprogramming is effective in combating obesity in humans ( Cheong and Xu, 2021 ). Better understanding the mechanisms by which obesity induces various medical consequences and identification and characterization of novel anti-obesity secreted factors/soluble molecules would be helpful for developing effective therapeutic treatments for obesity and its associated medical complications.
Bluher M. ( 2019 ). Obesity: global epidemiology and pathogenesis . Nat. Rev. Endocrinol . 15 , 288 – 298 .
Google Scholar
Cheong L.Y. , Xu A. ( 2021 ). Intercellular and inter-organ crosstalk in browning of white adipose tissue: molecular mechanism and therapeutic complications . J. Mol. Cell Biol . 13 , 466 – 479 .
Cho N.H. , Shaw J.E. , Karuranga S. , et al. ( 2018 ). IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045 . Diabetes Res. Clin. Pract . 138 , 271 – 281 .
Gao W. , Liu J.-L. , Lu X. , et al. ( 2021 ). Epigenetic regulation of energy metabolism in obesity . J. Mol. Cell Biol . 13 , 480 – 499 .
Huang B. , Xiong X. , Zhang L. , et al. ( 2021 ). PSA controls hepatic lipid metabolism by regulating the NRF2 signaling pathway . J. Mol. Cell Biol . 13 , 527 – 539 .
Loomba R. , Friedman S.L. , Shulman G.I. ( 2021 ). Mechanisms and disease consequences of nonalcoholic fatty liver disease . Cell 184 , 2537 – 2564 .
Love K.M. , Barrett E.J. , Malin S.K. , et al. ( 2021 ). Diabetes pathogenesis and management: the endothelium comes of age . J. Mol. Cell Biol . 13 , 500 – 512 .
Ruan H.-B. ( 2020 ). Developmental and functional heterogeneity of thermogenic adipose tissue . J. Mol. Cell Biol . 12 , 775 – 784 .
Xia X.-D. , Alabi A. , Wang M. , et al. ( 2021 ). Membrane-type I matrix metalloproteinase (MT1-MMP), lipid metabolism, and therapeutic implications . J. Mol. Cell Biol . 13 , 513 – 526 .
Author notes
Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Clinical Center for Diabetes, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai 200233, China E-mail: [email protected]
| Month: | Total Views: |
|---|---|
| October 2021 | 130 |
| November 2021 | 124 |
| December 2021 | 74 |
| January 2022 | 81 |
| February 2022 | 149 |
| March 2022 | 125 |
| April 2022 | 146 |
| May 2022 | 128 |
| June 2022 | 123 |
| July 2022 | 115 |
| August 2022 | 101 |
| September 2022 | 123 |
| October 2022 | 183 |
| November 2022 | 199 |
| December 2022 | 151 |
| January 2023 | 191 |
| February 2023 | 298 |
| March 2023 | 466 |
| April 2023 | 545 |
| May 2023 | 632 |
| June 2023 | 831 |
| July 2023 | 388 |
| August 2023 | 559 |
| September 2023 | 495 |
| October 2023 | 515 |
| November 2023 | 477 |
| December 2023 | 394 |
| January 2024 | 444 |
| February 2024 | 504 |
| March 2024 | 525 |
| April 2024 | 661 |
| May 2024 | 664 |
| June 2024 | 464 |
| July 2024 | 329 |
| August 2024 | 448 |
| September 2024 | 182 |
Email alerts
Citing articles via, affiliations.
- Online ISSN 1759-4685
- Copyright © 2024 Chinese Academy of Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- About Project
- Testimonials
Business Management Ideas

Essay on Obesity
List of essays on obesity, essay on obesity – short essay (essay 1 – 150 words), essay on obesity (essay 2 – 250 words), essay on obesity – written in english (essay 3 – 300 words), essay on obesity – for school students (class 5, 6, 7, 8, 9, 10, 11 and 12 standard) (essay 4 – 400 words), essay on obesity – for college students (essay 5 – 500 words), essay on obesity – with causes and treatment (essay 6 – 600 words), essay on obesity – for science students (essay 7 – 750 words), essay on obesity – long essay for medical students (essay 8 – 1000 words).
Obesity is a chronic health condition in which the body fat reaches abnormal level. Obesity occurs when we consume much more amount of food than our body really needs on a daily basis. In other words, when the intake of calories is greater than the calories we burn out, it gives rise to obesity.
Audience: The below given essays are exclusively written for school students (Class 5, 6, 7, 8, 9, 10, 11 and 12 Standard), college, science and medical students.
Introduction:
Obesity means being excessively fat. A person would be said to be obese if his or her body mass index is beyond 30. Such a person has a body fat rate that is disproportionate to his body mass.
Obesity and the Body Mass Index:
The body mass index is calculated considering the weight and height of a person. Thus, it is a scientific way of determining the appropriate weight of any person. When the body mass index of a person indicates that he or she is obese, it exposes the person to make health risk.
Stopping Obesity:
There are two major ways to get the body mass index of a person to a moderate rate. The first is to maintain a strict diet. The second is to engage in regular physical exercise. These two approaches are aimed at reducing the amount of fat in the body.
Conclusion:
Obesity can lead to sudden death, heart attack, diabetes and may unwanted illnesses. Stop it by making healthy choices.
Obesity has become a big concern for the youth of today’s generation. Obesity is defined as a medical condition in which an individual gains excessive body fat. When the Body Mass Index (BMI) of a person is over 30, he/ she is termed as obese.
Obesity can be a genetic problem or a disorder that is caused due to unhealthy lifestyle habits of a person. Physical inactivity and the environment in which an individual lives, are also the factors that leads to obesity. It is also seen that when some individuals are in stress or depression, they start cultivating unhealthy eating habits which eventually leads to obesity. Medications like steroids is yet another reason for obesity.
Obesity has several serious health issues associated with it. Some of the impacts of obesity are diabetes, increase of cholesterol level, high blood pressure, etc. Social impacts of obesity includes loss of confidence in an individual, lowering of self-esteem, etc.
The risks of obesity needs to be prevented. This can be done by adopting healthy eating habits, doing some physical exercise regularly, avoiding stress, etc. Individuals should work on weight reduction in order to avoid obesity.
Obesity is indeed a health concern and needs to be prioritized. The management of obesity revolves around healthy eating habits and physical activity. Obesity, if not controlled in its initial stage can cause many severe health issues. So it is wiser to exercise daily and maintain a healthy lifestyle rather than being the victim of obesity.
Obesity can be defined as the clinical condition where accumulation of excessive fat takes place in the adipose tissue leading to worsening of health condition. Usually, the fat is deposited around the trunk and also the waist of the body or even around the periphery.
Obesity is actually a disease that has been spreading far and wide. It is preventable and certain measures are to be taken to curb it to a greater extend. Both in the developing and developed countries, obesity has been growing far and wide affecting the young and the old equally.
The alarming increase in obesity has resulted in stimulated death rate and health issues among the people. There are several methods adopted to lose weight and they include different diet types, physical activity and certain changes in the current lifestyle. Many of the companies are into minting money with the concept of inviting people to fight obesity.
In patients associated with increased risk factor related to obesity, there are certain drug therapies and other procedures adopted to lose weight. There are certain cost effective ways introduced by several companies to enable clinic-based weight loss programs.
Obesity can lead to premature death and even cause Type 2 Diabetes Mellitus. Cardiovascular diseases have also become the part and parcel of obese people. It includes stroke, hypertension, gall bladder disease, coronary heart disease and even cancers like breast cancer, prostate cancer, endometrial cancer and colon cancer. Other less severe arising due to obesity includes osteoarthritis, gastro-esophageal reflux disease and even infertility.
Hence, serious measures are to be taken to fight against this dreadful phenomenon that is spreading its wings far and wide. Giving proper education on benefits of staying fit and mindful eating is as important as curbing this issue. Utmost importance must be given to healthy eating habits right from the small age so that they follow the same until the end of their life.
Obesity is majorly a lifestyle disease attributed to the extra accumulation of fat in the body leading to negative health effects on a person. Ironically, although prevalent at a large scale in many countries, including India, it is one of the most neglect health problems. It is more often ignored even if told by the doctor that the person is obese. Only when people start acquiring other health issues such as heart disease, blood pressure or diabetes, they start taking the problem of obesity seriously.
Obesity Statistics in India:
As per a report, India happens to figure as the third country in the world with the most obese people. This should be a troubling fact for India. However, we are yet to see concrete measures being adopted by the people to remain fit.
Causes of Obesity:
Sedentary lifestyle, alcohol, junk food, medications and some diseases such as hypothyroidism are considered as the factors which lead to obesity. Even children seem to be glued to televisions, laptops and video games which have taken away the urge for physical activities from them. Adding to this, the consumption of junk food has further aggravated the growing problem of obesity in children.
In the case of adults, most of the professions of today make use of computers which again makes people sit for long hours in one place. Also, the hectic lifestyle of today makes it difficult for people to spare time for physical activities and people usually remain stressed most of the times. All this has contributed significantly to the rise of obesity in India.
Obesity and BMI:
Body Mass Index (BMI) is the measure which allows a person to calculate how to fit he or she is. In other words, the BMI tells you if you are obese or not. BMI is calculated by dividing the weight of a person in kg with the square of his / her height in metres. The number thus obtained is called the BMI. A BMI of less than 25 is considered optimal. However, if a person has a BMI over 30 he/she is termed as obese.
What is a matter of concern is that with growing urbanisation there has been a rapid increase of obese people in India? It is of utmost importance to consider this health issue a serious threat to the future of our country as a healthy body is important for a healthy soul. We should all be mindful of what we eat and what effect it has on our body. It is our utmost duty to educate not just ourselves but others as well about this serious health hazard.
Obesity can be defined as a condition (medical) that is the accumulation of body fat to an extent that the excess fat begins to have a lot of negative effects on the health of the individual. Obesity is determined by examining the body mass index (BMI) of the person. The BMI is gotten by dividing the weight of the person in kilogram by the height of the person squared.
When the BMI of a person is more than 30, the person is classified as being obese, when the BMI falls between 25 and 30, the person is said to be overweight. In a few countries in East Asia, lower values for the BMI are used. Obesity has been proven to influence the likelihood and risk of many conditions and disease, most especially diabetes of type 2, cardiovascular diseases, sleeplessness that is obstructive, depression, osteoarthritis and some cancer types.
In most cases, obesity is caused through a combination of genetic susceptibility, a lack of or inadequate physical activity, excessive intake of food. Some cases of obesity are primarily caused by mental disorder, medications, endocrine disorders or genes. There is no medical data to support the fact that people suffering from obesity eat very little but gain a lot of weight because of slower metabolism. It has been discovered that an obese person usually expends much more energy than other people as a result of the required energy that is needed to maintain a body mass that is increased.
It is very possible to prevent obesity with a combination of personal choices and social changes. The major treatments are exercising and a change in diet. We can improve the quality of our diet by reducing our consumption of foods that are energy-dense like those that are high in sugars or fat and by trying to increase our dietary fibre intake.
We can also accompany the appropriate diet with the use of medications to help in reducing appetite and decreasing the absorption of fat. If medication, exercise and diet are not yielding any positive results, surgery or gastric balloon can also be carried out to decrease the volume of the stomach and also reduce the intestines’ length which leads to the feel of the person get full early or a reduction in the ability to get and absorb different nutrients from a food.
Obesity is the leading cause of ill-health and death all over the world that is preventable. The rate of obesity in children and adults has drastically increased. In 2015, a whopping 12 percent of adults which is about 600 million and about 100 million children all around the world were found to be obese.
It has also been discovered that women are more obese than men. A lot of government and private institutions and bodies have stated that obesity is top of the list of the most difficult and serious problems of public health that we have in the world today. In the world we live today, there is a lot of stigmatisation of obese people.
We all know how troubling the problem of obesity truly is. It is mainly a form of a medical condition wherein the body tends to accumulate excessive fat which in turn has negative repercussions on the health of an individual.
Given the current lifestyle and dietary style, it has become more common than ever. More and more people are being diagnosed with obesity. Such is its prevalence that it has been termed as an epidemic in the USA. Those who suffer from obesity are at a much higher risk of diabetes, heart diseases and even cancer.
In order to gain a deeper understanding of obesity, it is important to learn what the key causes of obesity are. In a layman term, if your calorie consumption exceeds what you burn because of daily activities and exercises, it is likely to lead to obesity. It is caused over a prolonged period of time when your calorie intake keeps exceeding the calories burned.
Here are some of the key causes which are known to be the driving factors for obesity.
If your diet tends to be rich in fat and contains massive calorie intake, you are all set to suffer from obesity.
Sedentary Lifestyle:
With most people sticking to their desk jobs and living a sedentary lifestyle, the body tends to get obese easily.
Of course, the genetic framework has a lot to do with obesity. If your parents are obese, the chance of you being obese is quite high.
The weight which women gain during their pregnancy can be very hard to shed and this is often one of the top causes of obesity.
Sleep Cycle:
If you are not getting an adequate amount of sleep, it can have an impact on the hormones which might trigger hunger signals. Overall, these linked events tend to make you obese.
Hormonal Disorder:
There are several hormonal changes which are known to be direct causes of obesity. The imbalance of the thyroid stimulating hormone, for instance, is one of the key factors when it comes to obesity.
Now that we know the key causes, let us look at the possible ways by which you can handle it.
Treatment for Obesity:
As strange as it may sound, the treatment for obesity is really simple. All you need to do is follow the right diet and back it with an adequate amount of exercise. If you can succeed in doing so, it will give you the perfect head-start into your journey of getting in shape and bidding goodbye to obesity.
There are a lot of different kinds and styles of diet plans for obesity which are available. You can choose the one which you deem fit. We recommend not opting for crash dieting as it is known to have several repercussions and can make your body terribly weak.
The key here is to stick to a balanced diet which can help you retain the essential nutrients, minerals, and, vitamins and shed the unwanted fat and carbs.
Just like the diet, there are several workout plans for obesity which are available. It is upon you to find out which of the workout plan seems to be apt for you. Choose cardio exercises and dance routines like Zumba to shed the unwanted body weight. Yoga is yet another method to get rid of obesity.
So, follow a blend of these and you will be able to deal with the trouble of obesity in no time. We believe that following these tips will help you get rid of obesity and stay in shape.
Obesity and overweight is a top health concern in the world due to the impact it has on the lives of individuals. Obesity is defined as a condition in which an individual has excessive body fat and is measured using the body mass index (BMI) such that, when an individual’s BMI is above 30, he or she is termed obese. The BMI is calculated using body weight and height and it is different for all individuals.
Obesity has been determined as a risk factor for many diseases. It results from dietary habits, genetics, and lifestyle habits including physical inactivity. Obesity can be prevented so that individuals do not end up having serious complications and health problems. Chronic illnesses like diabetes, heart diseases and relate to obesity in terms of causes and complications.
Factors Influencing Obesity:
Obesity is not only as a result of lifestyle habits as most people put it. There are other important factors that influence obesity. Genetics is one of those factors. A person could be born with genes that predispose them to obesity and they will also have difficulty in losing weight because it is an inborn factor.
The environment also influences obesity because the diet is similar in certain environs. In certain environments, like school, the food available is fast foods and the chances of getting healthy foods is very low, leading to obesity. Also, physical inactivity is an environmental factor for obesity because some places have no fields or tracks where people can jog or maybe the place is very unsafe and people rarely go out to exercise.
Mental health affects the eating habits of individuals. There is a habit of stress eating when a person is depressed and it could result in overweight or obesity if the person remains unhealthy for long period of time.
The overall health of individuals also matter. If a person is unwell and is prescribed with steroids, they may end up being obese. Steroidal medications enable weight gain as a side effect.
Complications of Obesity:
Obesity is a health concern because its complications are severe. Significant social and health problems are experienced by obese people. Socially, they will be bullied and their self-esteem will be low as they will perceive themselves as unworthy.
Chronic illnesses like diabetes results from obesity. Diabetes type 2 has been directly linked to obesity. This condition involves the increased blood sugars in the body and body cells are not responding to insulin as they should. The insulin in the body could also be inadequate due to decreased production. High blood sugar concentrations result in symptoms like frequent hunger, thirst and urination. The symptoms of complicated stages of diabetes type 2 include loss of vision, renal failure and heart failure and eventually death. The importance of having a normal BMI is the ability of the body to control blood sugars.
Another complication is the heightened blood pressures. Obesity has been defined as excessive body fat. The body fat accumulates in blood vessels making them narrow. Narrow blood vessels cause the blood pressures to rise. Increased blood pressure causes the heart to start failing in its physiological functions. Heart failure is the end result in this condition of increased blood pressures.
There is a significant increase in cholesterol in blood of people who are obese. High blood cholesterol levels causes the deposition of fats in various parts of the body and organs. Deposition of fats in the heart and blood vessels result in heart diseases. There are other conditions that result from hypercholesterolemia.
Other chronic illnesses like cancer can also arise from obesity because inflammation of body cells and tissues occurs in order to store fats in obese people. This could result in abnormal growths and alteration of cell morphology. The abnormal growths could be cancerous.
Management of Obesity:
For the people at risk of developing obesity, prevention methods can be implemented. Prevention included a healthy diet and physical activity. The diet and physical activity patterns should be regular and realizable to avoid strains that could result in complications.
Some risk factors for obesity are non-modifiable for example genetics. When a person in genetically predisposed, the lifestyle modifications may be have help.
For the individuals who are already obese, they can work on weight reduction through healthy diets and physical exercises.
In conclusion, obesity is indeed a major health concern because the health complications are very serious. Factors influencing obesity are both modifiable and non-modifiable. The management of obesity revolves around diet and physical activity and so it is important to remain fit.
In olden days, obesity used to affect only adults. However, in the present time, obesity has become a worldwide problem that hits the kids as well. Let’s find out the most prevalent causes of obesity.
Factors Causing Obesity:
Obesity can be due to genetic factors. If a person’s family has a history of obesity, chances are high that he/ she would also be affected by obesity, sooner or later in life.
The second reason is having a poor lifestyle. Now, there are a variety of factors that fall under the category of poor lifestyle. An excessive diet, i.e., eating more than you need is a definite way to attain the stage of obesity. Needless to say, the extra calories are changed into fat and cause obesity.
Junk foods, fried foods, refined foods with high fats and sugar are also responsible for causing obesity in both adults and kids. Lack of physical activity prevents the burning of extra calories, again, leading us all to the path of obesity.
But sometimes, there may also be some indirect causes of obesity. The secondary reasons could be related to our mental and psychological health. Depression, anxiety, stress, and emotional troubles are well-known factors of obesity.
Physical ailments such as hypothyroidism, ovarian cysts, and diabetes often complicate the physical condition and play a massive role in abnormal weight gain.
Moreover, certain medications, such as steroids, antidepressants, and contraceptive pills, have been seen interfering with the metabolic activities of the body. As a result, the long-term use of such drugs can cause obesity. Adding to that, regular consumption of alcohol and smoking are also connected to the condition of obesity.
Harmful Effects of Obesity:
On the surface, obesity may look like a single problem. But, in reality, it is the mother of several major health issues. Obesity simply means excessive fat depositing into our body including the arteries. The drastic consequence of such high cholesterol levels shows up in the form of heart attacks and other life-threatening cardiac troubles.
The fat deposition also hampers the elasticity of the arteries. That means obesity can cause havoc in our body by altering the blood pressure to an abnormal range. And this is just the tip of the iceberg. Obesity is known to create an endless list of problems.
In extreme cases, this disorder gives birth to acute diseases like diabetes and cancer. The weight gain due to obesity puts a lot of pressure on the bones of the body, especially of the legs. This, in turn, makes our bones weak and disturbs their smooth movement. A person suffering from obesity also has higher chances of developing infertility issues and sleep troubles.
Many obese people are seen to be struggling with breathing problems too. In the chronic form, the condition can grow into asthma. The psychological effects of obesity are another serious topic. You can say that obesity and depression form a loop. The more a person is obese, the worse is his/ her depression stage.
How to Control and Treat Obesity:
The simplest and most effective way, to begin with, is changing our diet. There are two factors to consider in the diet plan. First is what and what not to eat. Second is how much to eat.
If you really want to get rid of obesity, include more and more green vegetables in your diet. Spinach, beans, kale, broccoli, cauliflower, asparagus, etc., have enough vitamins and minerals and quite low calories. Other healthier options are mushrooms, pumpkin, beetroots, and sweet potatoes, etc.
Opt for fresh fruits, especially citrus fruits, and berries. Oranges, grapes, pomegranate, pineapple, cherries, strawberries, lime, and cranberries are good for the body. They have low sugar content and are also helpful in strengthening our immune system. Eating the whole fruits is a more preferable way in comparison to gulping the fruit juices. Fruits, when eaten whole, have more fibers and less sugar.
Consuming a big bowl of salad is also great for dealing with the obesity problem. A salad that includes fibrous foods such as carrots, radish, lettuce, tomatoes, works better at satiating the hunger pangs without the risk of weight gain.
A high protein diet of eggs, fish, lean meats, etc., is an excellent choice to get rid of obesity. Take enough of omega fatty acids. Remember to drink plenty of water. Keeping yourself hydrated is a smart way to avoid overeating. Water also helps in removing the toxins and excess fat from the body.
As much as possible, avoid fats, sugars, refined flours, and oily foods to keep the weight in control. Control your portion size. Replace the three heavy meals with small and frequent meals during the day. Snacking on sugarless smoothies, dry fruits, etc., is much recommended.
Regular exercise plays an indispensable role in tackling the obesity problem. Whenever possible, walk to the market, take stairs instead of a lift. Physical activity can be in any other form. It could be a favorite hobby like swimming, cycling, lawn tennis, or light jogging.
Meditation and yoga are quite powerful practices to drive away the stress, depression and thus, obesity. But in more serious cases, meeting a physician is the most appropriate strategy. Sometimes, the right medicines and surgical procedures are necessary to control the health condition.
Obesity is spreading like an epidemic, haunting both the adults and the kids. Although genetic factors and other physical ailments play a role, the problem is mostly caused by a reckless lifestyle.
By changing our way of living, we can surely take control of our health. In other words, it would be possible to eliminate the condition of obesity from our lives completely by leading a healthy lifestyle.
Health , Obesity
Get FREE Work-at-Home Job Leads Delivered Weekly!

Join more than 50,000 subscribers receiving regular updates! Plus, get a FREE copy of How to Make Money Blogging!
Message from Sophia!
Like this post? Don’t forget to share it!
Here are a few recommended articles for you to read next:
- Essay on Cleanliness
- Essay on Cancer
- Essay on AIDS
- Essay on Health and Fitness
No comments yet.
Leave a reply click here to cancel reply..
You must be logged in to post a comment.
Billionaires
- Donald Trump
- Warren Buffett
- Email Address
- Free Stock Photos
- Keyword Research Tools
- URL Shortener Tools
- WordPress Theme
Book Summaries
- How To Win Friends
- Rich Dad Poor Dad
- The Code of the Extraordinary Mind
- The Luck Factor
- The Millionaire Fastlane
- The ONE Thing
- Think and Grow Rich
- 100 Million Dollar Business
- Business Ideas
Digital Marketing
- Mobile Addiction
- Social Media Addiction
- Computer Addiction
- Drug Addiction
- Internet Addiction
- TV Addiction
- Healthy Habits
- Morning Rituals
- Wake up Early
- Cholesterol
- Reducing Cholesterol
- Fat Loss Diet Plan
- Reducing Hair Fall
- Sleep Apnea
- Weight Loss
Internet Marketing
- Email Marketing
Law of Attraction
- Subconscious Mind
- Vision Board
- Visualization
Law of Vibration
- Professional Life
Motivational Speakers
- Bob Proctor
- Robert Kiyosaki
- Vivek Bindra
- Inner Peace
Productivity
- Not To-do List
- Project Management Software
- Negative Energies
Relationship
- Getting Back Your Ex
Self-help 21 and 14 Days Course
Self-improvement.
- Body Language
- Complainers
- Emotional Intelligence
- Personality
Social Media
- Project Management
- Anik Singal
- Baba Ramdev
- Dwayne Johnson
- Jackie Chan
- Leonardo DiCaprio
- Narendra Modi
- Nikola Tesla
- Sachin Tendulkar
- Sandeep Maheshwari
- Shaqir Hussyin
Website Development
Wisdom post, worlds most.
- Expensive Cars
Our Portals: Gulf Canada USA Italy Gulf UK
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making (2010)
Chapter: 10 conclusions and recommendations, 10 conclusions and recommendations.
D ecisions about prevention are complex, not only for the obesity problem but also for other problems with multiple types and layers of causation. Recognition of the need to emphasize population-based approaches to obesity prevention, the urgency of taking action, and the desire of many decision makers to have evidence on which actions to take have created a demand for evidence with which to answer a range of questions. In reality, the evidence approaches that apply to decision making about the treatment of obesity or other clinical problems are inadequate and sometimes inappropriate for application to decisions about public health initiatives. The need to work around evidence gaps and the limitations of using evidence hierarchies that apply to medical treatment for assessing population-based preventive interventions have been faced by the developers of several prior Institute of Medicine (IOM) reports on obesity prevention (focused on child and adolescent obesity). These evidence issues are not new and have already been the focus of many efforts in the field of public health in relation to other complex health problems. However, they are far from resolved. Considering these issues in relation to obesity prevention has the potential to advance the field of public health generally while also meeting the immediate need for clarity on evidence issues related to addressing the obesity epidemic.
The IOM’s Food and Nutrition Board formed the Committee on an Evidence Framework for Obesity Prevention Decision Making, with funding from Kaiser Permanente, the Robert Wood Johnson Foundation, and the Centers for Disease Control and Prevention. This committee was asked to develop a framework for evidence-informed decision making in obesity prevention, focused on approaches for assessing policy, environmental, and community interventions designed to influence diet and physical activity. The committee was tasked to:
provide an overview of the nature of the evidence base for obesity prevention as it is currently construed;
identify the challenges associated with integrating scientific evidence with broader influences on policy and programmatic considerations;
provide a practical and action-oriented framework of recommendations for how to select, implement, and evaluate obesity prevention efforts;
identify ways in which existing or new tools and methods can be used to build a useful and timely evidence base appropriate to the challenges presented by the epidemic, and describe ongoing attempts to meet these challenges;
develop a plan for communicating and disseminating the proposed framework and its recommendations; and
specify a plan for evaluating and refining the proposed framework in current decision-making processes.
CONCLUSIONS
Recognition is increasing that overweight and obesity are not only problems of individuals, but also societywide problems of populations. Acting on this recognition will require multifaceted, population-based changes in the socioenvironmental variables that influence energy intake and expenditure. There exist both a pressing need to act on the problem of obesity and a large gap between the type and amount of evidence needed to act and the type and amount of evidence available to meet that need. A new framework is necessary to assist researchers and a broad community of decision makers in generating, identifying, and evaluating the best evidence available and in summarizing it for use in decision making. This new framework also is important for researchers attempting to fill important evidence gaps through studies based on questions with program and policy relevance. However, the methods used and the evidence generated by traditional research designs do not yield all the types of evidence useful to inform actions aimed at addressing obesity prevention and other complex public health challenges. An expanded approach is needed that emphasizes the decision-making process and contextual considerations.
The Framework
To meet this need, the committee developed the L.E.A.D. ( L ocate Evidence, E valuate Evidence, A ssemble Evidence, and Inform D ecisions) framework, designed to facilitate a systematic approach to the identification, implementation, and evaluation of promising, reasonable actions to address obesity prevention and other complex public health challenges (see Figure 10-1 ). The framework is designed to help identify the nature of the evidence that is needed and clarify what changes in current approaches to generating and evaluating evidence will facilitate meeting those needs. This section describes the main components of the framework and issues related to these components.
Obesity prevention has not been addressed successfully by traditional study designs, which are generally linear and static. A systems approach is needed to develop more complex, interdisciplinary strategies. Accordingly, the L.E.A.D. framework

FIGURE 10-1 The L ocate Evidence, E valuate Evidence, A ssemble Evidence, Inform D ecisions (L.E.A.D.) framework for obesity prevention decision making.
recommends taking a systems perspective. In other words, it is necessary to use an approach that encompasses the whole picture, highlighting the broader context and interactions among levels, to capture the complexity of obesity prevention and other multifactorial public health challenges.
Addressing such challenges first requires specifying the question(s) being asked to guide the identification of evidence that is appropriate, inclusive, and relevant. Core to the framework is the orientation of the user. A variety of decisions have to be made to address obesity prevention. To capture the resulting mix of evidence needs, the framework adopts a typology that differentiates three broad categories of interrelated questions of potential interest to the user: Why should we do something about this problem? What specifically should we do? and How do we implement this information for our situation? This “Why,” “What,” “How” typology stresses the need for multiple types of evidence to support decisions on obesity prevention.
Once the question(s) of interest have been specified, locating useful evidence requires clear knowledge of the types of information that may be useful and an awareness of where that information can be found. The framework calls for the use of
diverse approaches to gather and synthesize information from other disciplines that address issues similar to those faced in obesity prevention and public health generally. Evidence identified and gathered to inform decision making for obesity prevention and other complex public health challenges should be assessed based on both its generalizability and level of certainty (i.e., its external and internal validity, respectively). The L.E.A.D. framework addresses these two key aspects of the evidence through the nature of the question(s) being asked, established criteria for the value of evidence, and the context in which the question(s) arise. Results of the overall evaluation of evidence should provide answers on what to do, how to do it, and how strongly the action is justified.
When decision makers are coming to a decision on obesity prevention actions, it is important for them to understand the state of the available knowledge relevant to that decision. This knowledge includes evidence on the specific problem to be addressed, the likely effectiveness and impact of proposed actions, and key considerations involved in their implementation. Successful evidence gathering, evaluation, and synthesis for use in obesity prevention usually require the involvement of a number of disciplines using a variety of methodologies and technical languages. The framework incorporates a standardized approach using a uniform language and structure for summarizing the relevant evidence in a systematic, transparent, and transdisciplinary way that is critical for communicating the process and conclusions clearly.
With an emergent problem such as obesity, decisions to act often must be made in the face of a relative absence of evidence, or evidence that is inconclusive, inconsistent, or incomplete. Evidence gathered from a particular intervention implemented in a closely controlled manner within a specific population with its own unique characteristics is often difficult to apply to a similar intervention with another population. The typical way of presenting results of obesity prevention efforts in journals often adds to the problem of incomplete evidence because useful aspects of the research related to its generalizability are not reported. If obesity prevention actions must be taken when evidence is limited, this incomplete evidence can be blended with theory, expert opinion, experience, and local wisdom to make the best decision possible. The actions taken then should undergo critical evaluation, the results of which should be used to build credible evidence for use in decision making about future efforts. Important alternatives to waiting for the funding, implementation, and publication of formal research on obesity prevention are natural experiments as sources of practice-based evidence, “evaluability assessment” of emerging innovations (defined as assessing whether a program is ready for full-scale evaluation), and continuous quality assessment of ongoing programs. The L.E.A.D. framework process leads to knowledge integration, or the incorporation of new knowledge gained through the process of applying the framework into the context of the organization or system where decisions are made.
The evidence base to support the identification of effective obesity prevention interventions is limited in many areas. Opportunities to generate evidence may occur
at any phase of the evidence review or decision-making process. The L.E.A.D. framework guides the generation of evidence related to “What,” “Why,” and “How” questions and supports the use of multiple forms of evidence and research designs from a variety of disciplines. In obesity prevention–related research, the generation of evi dence from evaluation of ongoing and emerging initiatives is a particular priority.
Researchers, decision makers, and intermediaries working on obesity prevention and other complex multifactorial public health problems are the primary audiences for communicating and disseminating the L.E.A.D. framework. With sufficient information, they can apply the framework as a guide for generating needed evidence and supporting decision making. It is important to understand the settings, communication channels, and activities of these key audiences to engage and educate them effectively on the purpose and adoption of the framework. To support the development of a communication and dissemination plan, it is critical to create partnerships, make use of existing activities and networks, and tailor the messages and approaches to each target audience.
As the target audiences begin to use the framework, assessing its use in selected settings will be essential so it can be improved and refined. Evaluation of the impact of the L.E.A.D. framework is also important for determining its relevance to current evidence-generation and decision-making processes. To this end, key outcome measures—utilization, adoption, acceptance, maintenance, and impact—should be defined and data collected on these measures. It will be important to develop or adopt data collection tools and utilize methods and existing initiatives that will best serve this purpose, as well as to systematically integrate the feedback thus obtained to sustain and improve the framework’s applicability and utilization.
RECOMMENDATIONS
The United States has made progress toward translating science into practice in the brief time since the obesity epidemic was officially recognized. But the pace of this translation has been slow relative to the scope and urgency of the problem and the associated harms and costs. As discussed above, moreover, the evidence emerging from applied research on obesity prevention can be inconclusive, incomplete, and inconsistent. A systematic process is needed to improve the use of available evidence and increase and enhance the evidence base to inform decisions on obesity prevention and other complex public health problems. Commitment to such a process is needed from both decision makers and those involved in generating evidence, including public and private policy makers and their advisors, scientific and policy think tanks, advocacy groups and stakeholders, program planners, practitioners in public health and other sectors, program evaluators, public health researchers and research scientists, journal editors, and funders. With this in mind, the committee makes the following recom-
mendations for assisting decision makers and researchers in using the current evidence base for obesity prevention and for taking a systems-oriented, transdisciplinary approach to generate more, and more useful, evidence.
Utilize the L.E.A.D. Framework
Recommendation 1: Decision makers and those involved in generating evidence, including researchers, research funders, and publishers of research, should apply the L.E.A.D. framework as a guide in their utilization and generation of evidence to support decision making for complex, multifactorial public health challenges, including obesity prevention.
Key assumptions that should guide the use of the framework include the following:
A systems perspective can help in framing and explaining complex issues.
The types of evidence that should be gathered to inform decision making are based on the nature of the questions being asked, including Why? (“Why should we do something about this problem in our situation?”), What? (“What specifically should we do about this problem?”), and How? (“How do we implement this information for our situation?”). A focus on subsets of these questions as a starting point in gathering evidence explicitly expands the evidence base that is typically identified and gathered.
The quality of the evidence should be judged according to established criteria for that type of evidence.
Both the level of certainty of the causal relationship between an intervention and the observed outcomes and the intervention’s generalizability to other individuals, settings, contexts, and time frames should be given explicit attention.
The analysis of the evidence to be used in making a decision should be summarized and communicated in a systematic, transparent, and transdisciplinary manner that uses uniform language and structure. The report on this analysis should include a summary of the question(s) asked by the decision maker; the strategy for gathering and selecting the evidence; an evidence table showing the sources, types, and quality of the evidence and the outcomes reported; and a concise summary of the synthesis of selected evidence on why an action should be taken, what that action should be, and how it should be taken.
If action must be taken when evidence is limited, this incomplete evidence can be blended carefully and transparently with theory, expert opinion, and collaboration based on professional experience and local wisdom to support making the best decision.
Sustained commitments will be needed from both the public and private sectors to achieve successful utilization of the various elements of the L.E.A.D. framework in future evidence-informed decision making and evidence generation. This respon-
sibility lies with the academic and research community, as well as with government and private funders and the leadership of journals that publish research in this area. Necessary supports will include increasing understanding of systems thinking and incorporating it into research-related activities, creating and maintaining resources to support the utilization of evidence, establishing standards of quality for different types of evidence, and supporting the generation of evidence, each of which is described in more detail below. Finally, it will be necessary to communicate, disseminate, evaluate, and refine the L.E.A.D. framework.
Incorporate Systems Thinking
Recommendation 2: Researchers, government and private funders, educators, and journal editors should incorporate systems thinking into their research-related activities.
To implement this recommendation:
Researchers should use systems thinking to guide the development of environmental and policy interventions and study designs.
Government and private funders should encourage the use of systems thinking in their requests for proposals and include systems considerations in proposal evaluations.
Universities, government agencies such as the U.S. Centers for Disease Control and Prevention, and public health organizations responsible for educating public health practitioners and related researchers should establish training capacity for the science and understanding of systems thinking and the use of systems mapping and other quantitative or qualitative systems analysis tools.
Journal editors should encourage the use of systems thinking for addressing complex problems by developing panels of peer reviewers with expertise in this area and charging them with making recommendations for how authors could use systems thinking more effectively in their manuscripts.
Build a Resource Base
Recommendation 3: Government, foundations, professional organizations, and research institutions should build a system of resources (people, compendiums of knowledge, registries of implementation experience) to support evidence-based public policy decision making and research for complex health challenges, including obesity prevention.
The Secretary of Health and Human Services, in collaboration with other public- and private-sector partners, should establish a sustainable registry of reports on evidence for environmental and policy actions for obesity prevention.
Integral to this registry should be the expanded view of evidence for decision making on obesity prevention proposed in this report and the sharing of experiences and innovative programs as the evidence evolves. A service provided by this registry should be periodic synthesis reviews based on mixed qualitative and quantitative methods.
The Secretary of Health and Human Services, in collaboration with other public- and private-sector partners, should develop and fund a resource for compiling and linking existing databases that may contain useful evidence for obesity prevention and related public health initiatives. This resource should include links to data and research from disciplines and sectors outside of obesity prevention and public health and to data from nonacademic sources that are of interest to decision makers.
Establish Standards for Evidence Quality
Recommendation 4: Government, foundations, professional organizations, and research institutions should catalyze and support the establishment of guidance on standards for evaluating the quality of evidence for which such standards are lacking.
Government and private funders should give priority to funding for the development of guidance on standards for evaluating the quality of the full range of evidence types discussed in this report that are useful in making obesity prevention decisions, especially those for which the scientific literature is limited.
Professional organizations and research institutions should encourage and bring attention to efforts by faculty, researchers, and students to establish guidance in this area.
Support the Generation of Evidence
Recommendation 5: Obesity prevention research funders, researchers, and publishers should consider, wherever appropriate, the inclusion in research studies of a focus on the generalizability of the find ings and related implementation issues at every stage, from conception through publication.
Those funding research in obesity prevention should give priority to support for studies that include an assessment of the limitations, potential utility, and applicability of the research beyond the particular population, setting, and circumstances in which the studies are conducted, including by initiating requests for applications and similar calls for proposals aimed at such studies. Additional ways in which this recommendation could be implemented include adding crite-
ria related to generalizability to proposal review procedures and training reviewers to evaluate generalizability.
Obesity prevention researchers and program evaluators should give special consideration to study designs that maximize evidence on generalizability.
Journal editors should provide guidelines and space for authors to give richer descriptions of interventions and the conditions under which they are tested to clarify their generalizability.
Recommendation 6: Research funders should increase opportunities for those carrying out obesity pre vention initiatives to measure and share their outcomes so others can learn from their experience.
Organizations funding or sponsoring obesity prevention initiatives—including national, regional, statewide, or local programs; policy changes; and environmental initiatives—should provide resources for obtaining practice-based evidence from innovative and ongoing programs and policies in a more routine, timely, and systematic manner to capture their processes, implementation, and outcomes. These funders should also encourage and support assessments of the potential for evaluating the most innovative programs in their jurisdictions and sponsor scientific evaluations where the opportunities to advance generalizable evidence are greatest.
Research funders, researchers, and journal editors should assign higher priority to studies that test obesity prevention interventions in real-world settings in which major contextual variables are identified and their influence is evaluated.
Recommendation 7: Research funders should encourage collaboration among researchers in a variety of disciplines so as to utilize a full range of research designs that may be feasible and appropriate for evaluating obesity prevention and related public health initiatives.
As part of their requests for proposals on obesity prevention research, funders should give priority to and reward transdisciplinary collaborations that include the creative use of research designs that have not been extensively used in prevention research but hold promise for expanding the evidence base on potential environmental and policy solutions.
Communicate, Disseminate, Evaluate, and Refine the L.E.A.D. Framework
Recommendation 8: A public–private consortium should bring together researchers, research funders, publishers of research, decision makers, and other stakeholders to discuss the practical uses of the
L.E.A.D. framework, and develop plans and a timeline for focused experimentation with the frame work and for its evaluation and potential refinement.
Interested funders should bring together a consortium of representatives of key stakeholders (including decision makers, government funders, private funders, academic institutions, professional organizations, researchers, and journal editors) who are committed to optimizing the use of the current obesity prevention evidence base and developing a broader and deeper base of evidence.
This consortium should develop an action-oriented plan for funding and implementing broad communication, focused experimentation, evaluation, and refinement of the L.E.A.D. framework. This plan should be based on the major purposes of the framework: to significantly improve the evidence base for obesity prevention decision making on policy and environmental solutions, and to assist decision makers in using the evidence base.
To battle the obesity epidemic in America, health care professionals and policymakers need relevant, useful data on the effectiveness of obesity prevention policies and programs. Bridging the Evidence Gap in Obesity Prevention identifies a new approach to decision making and research on obesity prevention to use a systems perspective to gain a broader understanding of the context of obesity and the many factors that influence it.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 23 September 2021
The genetics of obesity: from discovery to biology
- Ruth J. F. Loos ORCID: orcid.org/0000-0002-8532-5087 1 , 2 , 3 , 4 &
- Giles S. H. Yeo ORCID: orcid.org/0000-0001-8823-3615 5
Nature Reviews Genetics volume 23 , pages 120–133 ( 2022 ) Cite this article
158k Accesses
466 Citations
484 Altmetric
Metrics details
- Disease genetics
- Endocrine system and metabolic diseases
- Genetic association study
- Genetic variation
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people’s health. Polygenic (or common) obesity and rare, severe, early-onset monogenic obesity are often polarized as distinct diseases. However, gene discovery studies for both forms of obesity show that they have shared genetic and biological underpinnings, pointing to a key role for the brain in the control of body weight. Genome-wide association studies (GWAS) with increasing sample sizes and advances in sequencing technology are the main drivers behind a recent flurry of new discoveries. However, it is the post-GWAS, cross-disciplinary collaborations, which combine new omics technologies and analytical approaches, that have started to facilitate translation of genetic loci into meaningful biology and new avenues for treatment.
Similar content being viewed by others
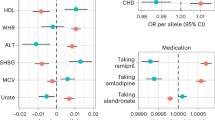
A phenome-wide comparative analysis of genetic discordance between obesity and type 2 diabetes
Obesity: exploring its connection to brain function through genetic and genomic perspectives

Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities
Introduction.
Obesity is associated with premature mortality and is a serious public health threat that accounts for a large proportion of the worldwide non-communicable disease burden, including type 2 diabetes, cardiovascular disease, hypertension and certain cancers 1 , 2 . Mechanical issues resulting from substantially increased weight, such as osteoarthritis and sleep apnoea, also affect people’s quality of life 3 . The impact of obesity on communicable disease, in particular viral infection 4 , has recently been highlighted by the discovery that individuals with obesity are at increased risk of hospitalization and severe illness from COVID-19 (refs 5 , 6 , 7 ).
On the basis of the latest data from the NCD Risk Factor Collaboration, in 2016 almost 2 billion adults (39% of the world’s adult population) were estimated to be overweight (defined by a body mass index (BMI) of ≥25 kg m − 2 ), 671 million (12% of the world’s adult population) of whom had obesity (BMI ≥30 kg m − 2 ) — a tripling in the prevalence of obesity since 1975 (ref. 8 ) (Fig. 1 ). Although the rate of increase in obesity seems to be declining in most high-income countries, it continues to rise in many low-income and middle-income countries and prevalence remains high globally 8 . If current trends continue, it is expected that 1 billion adults (nearly 20% of the world population) will have obesity by 2025. Particularly alarming is the global rise in obesity among children and adolescents; more than 7% had obesity in 2016 compared with less than 1% in 1975 (ref. 8 ).
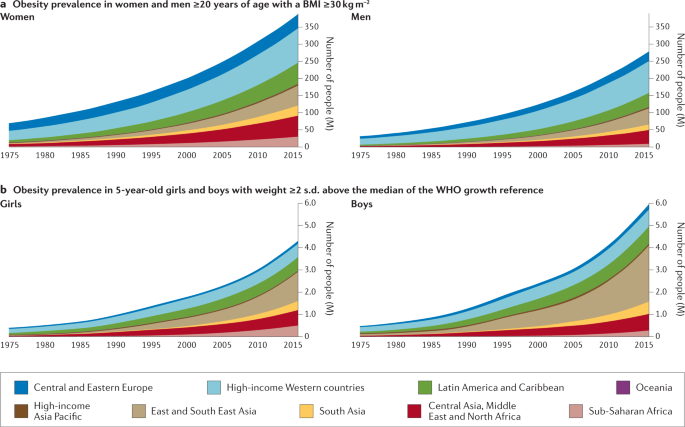
The prevalence of obesity has risen steadily over the past four decades in children, adolescents (not shown) and adults worldwide. a | Prevalence of obesity (body mass index (BMI) ≥30 kg m −2 ) in women and men ≥20 years of age, from 1975 to 2016. b | Prevalence of obesity (weight ≥2 s.d. above the median of the WHO growth reference) in 5-year-old girls and boys from 1975 to 2016. Geographical regions are represented by different colours. Graphs are reproduced from the NCD Risk Factor Collaboration (NCD RisC) website and are generated from data published in ref. 8 .
Although changes in the environment have undoubtedly driven the rapid increase in prevalence, obesity results from an interaction between environmental and innate biological factors. Crucially, there is a strong genetic component underlying the large interindividual variation in body weight that determines people’s response to this ‘obesogenic’ environment . Twin, family and adoption studies have estimated the heritability of obesity to be between 40% and 70% 9 , 10 . As a consequence, genetic approaches can be leveraged to characterize the underlying physiological and molecular mechanisms that control body weight.
Classically, we have considered obesity in two broad categories (Fig. 2 ): so-called monogenic obesity , which is inherited in a Mendelian pattern, is typically rare, early-onset and severe and involves either small or large chromosomal deletions or single-gene defects; and polygenic obesity (also known as common obesity), which is the result of hundreds of polymorphisms that each have a small effect. Polygenic obesity follows a pattern of heritability that is similar to other complex traits and diseases. Although often considered to be two distinct forms, gene discovery studies of monogenic and polygenic obesity have converged on what seems to be broadly similar underlying biology. Specifically, the central nervous system (CNS) and neuronal pathways that control the hedonic aspects of food intake have emerged as the major drivers of body weight for both monogenic and polygenic obesity. Furthermore, early evidence shows that the expression of mutations causing monogenic obesity may — at least in part — be influenced by the individual’s polygenic susceptibility to obesity 11 .
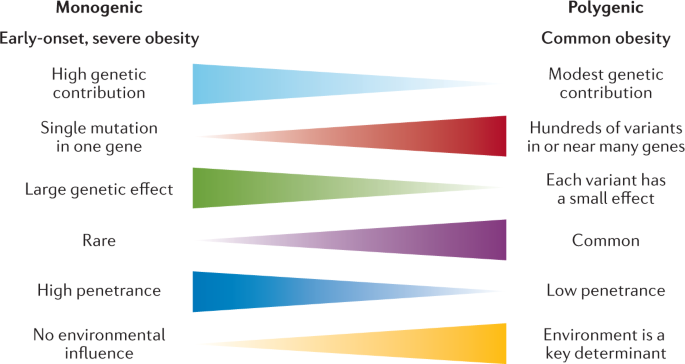
Key features of monogenic and polygenic forms of obesity .
In this Review, we summarize more than 20 years of genetic studies that have characterized the molecules and mechanisms that control body weight, specifically focusing on overall obesity and adiposity, rather than fat distribution or central adiposity. Although most of the current insights into the underlying biology have been derived from monogenic forms of obesity, recent years have witnessed several successful variant-to-function translations for polygenic forms of obesity. We also explore how the ubiquity of whole-exome sequencing (WES) and genome sequencing has begun to blur the line that used to demarcate the monogenic causes of obesity from common polygenic obesity. Syndromic forms of obesity, such as Bardet–Biedl, Prader–Willi, among many others 12 , are not reviewed here. Although obesity is often a dominant feature of these syndromes, the underlying genetic defects are often chromosomal abnormalities and typically encompass multiple genes, making it difficult to decipher the precise mechanisms directly related to body-weight regulation. Finally, as we enter the post-genomic era, we consider the prospects of genotype-informed treatments and the possibility of leveraging genetics to predict and hence prevent obesity.
Gene discovery approaches
The approaches used to identify genes linked to obesity depend on the form of obesity and genotyping technology available at the time. Early gene discovery studies for monogenic forms of obesity had a case-focused design: patients with severe obesity, together with their affected and unaffected family members, were examined for potential gene-disrupting causal mutations via Sanger sequencing. By contrast, genetic variation associated with common forms of obesity have been identified in large-scale population studies, either using a case–control design or continuous traits such as BMI. Gene discovery for both forms of obesity was initially hypothesis driven; that is, restricted to a set of candidate genes that evidence suggests have a role in body-weight regulation. Over the past two decades, however, advances in high-throughput genome-wide genotyping and sequencing technologies, combined with a detailed knowledge of the human genetic architecture, have enabled the interrogation of genetic variants across the whole genome for their role in body-weight regulation using a hypothesis-generating approach.
Gene discovery for monogenic obesity
Many of the candidate genes and pathways linked to body-weight regulation were initially identified in mice, such as the obese ( ob ) 13 and diabetes ( db ) 14 mouse lines, in which severe hyperphagia and obesity spontaneously emerged. Using reverse genetics , the ob gene was shown to encode leptin, a hormone produced from fat, and it was demonstrated that leptin deficiency resulting from a mutation in the ob gene caused the severe obesity seen in the ob/ob mouse 15 (Fig. 3 ). Shortly after the cloning of ob , the db gene was cloned and identified as encoding the leptin receptor (LEPR) 16 . Reverse genetics was also used to reveal that the complex obesity phenotype of Agouti ‘lethal yellow’ mice is caused by a rearrangement in the promoter sequence of the agouti gene that results in ectopic and constitutive expression of the agouti peptide 17 , 18 , which antagonizes the melanocortin 1 and 4 receptors (MC1R and MC4R) 19 , 20 . This finding linked the melanocortin pathway to body-weight regulation, thereby unveiling a whole raft of new candidate genes for obesity.
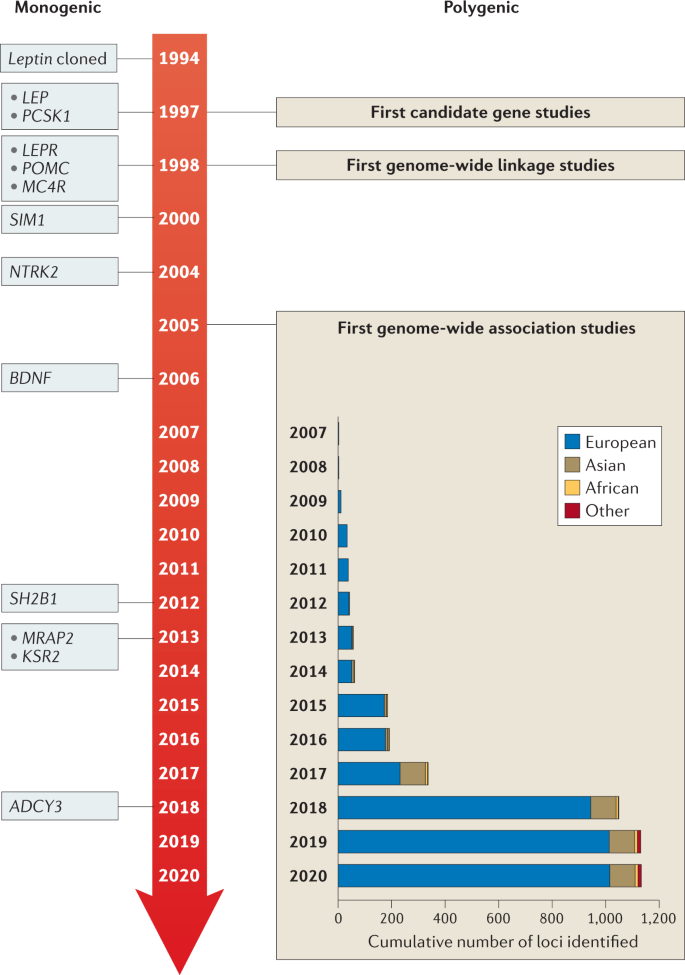
Genes identified for monogenic obesity in a given year are shown on the left. Discoveries made for polygenic obesity are shown on the right, including a cumulative count of newly discovered loci per year and by ancestry. Although candidate gene and genome-wide linkage studies became available in the late 1990s, findings were limited, and these study designs are not as frequently used as genome-wide association studies.
Once the genes for leptin and its receptor were identified, they became candidate genes for human obesity, and in 1997 the first humans with congenital leptin deficiency were identified 21 . This discovery was rapidly followed by the report of humans with mutations in the gene encoding the leptin receptor ( LEPR ) 22 , as well as in genes encoding multiple components of the melanocortin pathway, including PCSK1 (ref. 23 ), MC4R 24 , 25 , 26 and POMC 27 , 28 , 29 , all of which were found to result in severe early-onset obesity (Table 1 ).
Advances in high-throughput DNA sequencing led to candidate gene screening being replaced by WES, an unbiased approach that allows all coding sequences to be screened for mutations. However, it rapidly became clear that, whereas candidate gene studies yielded few mutations, WES identified too many potential obesity-associated variants such that the noise often masked the true causative mutations. However, with improved algorithms to predict the pathogenicity of mutations, as well as a rapidly expanding toolkit of functional assays, it has become easier to filter the likely pathogenic mutations. Several success stories have been reported in which WES has identified novel pathways and genes linked to obesity, such as the class 3 semaphorins (SEMA3A–G), which have been shown to direct the development of certain hypothalamic neurons, including those expressing pro-opiomelanocortin (POMC) 30 (see ‘Other neuronal circuits and molecules linked to severe obesity’).
Most monogenic obesity mutations have been identified in cohorts of patients with severe and early-onset (<10 years old) obesity. Additionally, as monogenic obesity often demonstrates a recessive inheritance pattern 31 , consanguinity in populations has further increased the chance of identifying mutations, owing to greater chances of homozygosity of deleterious mutations 32 . For example, studies have reported that mutations in the genes encoding leptin, LEPR and MC4R explain 30% of cases of severe obesity in children from a consanguineous Pakistani population 33 , and single-gene defects more broadly account for nearly 50% 34 .
Gene discovery for polygenic obesity
The discovery of genes that influence polygenic obesity, which is common in the general population, started off slowly with candidate gene studies and genome-wide linkage studies . The candidate gene approach was first applied in the mid-1990s and aimed to validate genes identified through human and animal models of extreme obesity for a role in common obesity (Fig. 3 ). Common variants in such candidate genes were tested for association with obesity risk, BMI or other body composition traits. Over the subsequent 15 years, hundreds of genes were studied as candidates, but variants in only six ( ADRB3 (ref. 35 ), BDNF 36 , CNR1 (ref. 37 ), MC4R 38 , PCSK1 (ref. 39 ) and PPARG 40 ) showed reproducible association with obesity outcomes. The genome-wide linkage approach made its entrance into the field towards the end of the 1990s (Fig. 3 ). Genome-wide linkage studies rely on the relatedness of individuals and test whether certain chromosomal regions co-segregate with a disease or trait across generations. Even though more than 80 genome-wide linkage studies identified >300 chromosomal loci with suggestive evidence of linkage with obesity traits, few loci were replicated and none was successfully fine-mapped to pinpoint the causal gene or genes 41 . Ultimately, candidate gene and genome-wide linkage studies, constrained by small sample sizes, sparse coverage of genetic variation across the genome and lack of replication, only had a marginal impact on the progression of gene discovery for common obesity outcomes.
However, the pace of gene discovery for common diseases accelerated with the advent of genome-wide association studies (GWAS) (Fig. 3 ). The first GWAS for obesity traits were published in 2007 and identified a cluster of common variants in the first intron of the FTO locus that was convincingly associated with BMI 42 , 43 . Many more GWAS followed and, to date, nearly 60 GWAS have identified more than 1,100 independent loci associated with a range of obesity traits 44 (Supplementary Tables 1 , 2 ).
As sample sizes increase with each consecutive GWAS, the statistical power to identify more loci also increases, in particular for loci that are less common and/or have smaller effects. For example, the first GWAS were relatively small ( n = ~5,000) and identified only the FTO locus 42 , 43 . The BMI-increasing allele of FTO is common, particularly in populations of European ancestry (minor allele frequency (MAF) 40–45%), and has a relatively large effect on BMI (0.35 kg m −2 per allele; equivalent to 1 kg for a person who is 1.7 m tall). Ten years and numerous GWAS later, the most recent GWAS for BMI included nearly 800,000 individuals, identified more than 750 loci, with MAFs as small as 1.6% and per-allele effects as low as 0.04 kg m −2 per allele (equivalent to 120 g for a person who is 1.7 m tall) 45 . Combined, these genome-wide significant loci explained 6% of variation in BMI 45 . Large-scale international collaborations have been formed, such as the Genetic Investigation for Anthropometric Traits (GIANT) consortium , that combine summary statistics of individual GWAS to generate data sets comprising hundreds of thousands of individuals. Furthermore, many GWAS efforts have maximized sample size by focusing on BMI as the primary obesity outcome, an inexpensive and easy-to-obtain measurement that is readily available in most studies. As such, the vast majority of loci have been identified first in GWAS of BMI, but their effects typically transfer to other overall adiposity outcomes.
Even though BMI is widely used, it is considered a crude proxy of overall adiposity because it does not distinguish between lean and fat mass 46 . Therefore, GWAS have been performed for more refined obesity traits, such as body fat percentage 47 , 48 , fat-free mass 49 , imaging-derived adipose tissue 50 , circulating leptin levels 51 and LEPR levels 52 . In addition, two GWAS have focused on persistent healthy thinness, assuming that genes that determine resistance to weight gain may also inform obesity prevention and weight loss maintenance 53 , 54 . Although GWAS of more refined and alternative obesity outcomes are generally much smaller than those for BMI, the phenotypes are often a more accurate representation of body-weight regulation and, as such, the loci identified tend to more often point to relevant biological pathways that underlie obesity.
Almost all GWAS loci for obesity outcomes were first identified in adults. Most of these loci also associate with obesity and/or BMI in children and adolescents, highlighting the fact that the genetic underpinning of obesity is relatively constant across the course of life 55 , 56 , 57 . Similarly to gene discovery for other common diseases, the obesity genetics field has suffered from a strong bias in population representation, with the vast majority of GWAS being performed in populations that are exclusively or predominantly of European ancestry. Nevertheless, some loci have first been discovered in populations of Asian 58 , African 59 , 60 , Hispanic or other ancestry 61 , despite their much smaller sample sizes. Broadly, loci identified in one ancestry demonstrate good transferability (that is, directionally consistent associations) across other ancestries, even though effect sizes and allele frequencies may differ. The modest-to-high genetic correlations across ancestries observed for BMI ( r = 0.78) are consistent with good transferability 62 , but also suggest that ancestry-specific loci remain to be discovered. Besides increasing the sample sizes of GWAS in populations of non-European ancestry, demographic, evolutionary and/or genomic features of specific populations (such as founder, consanguineous or isolated populations) have been leveraged for gene discovery, identifying genetic variants with large effects that are common in the discovery population, such as CREBRF , first identified in Samoan populations, and ADCY3 , first identified in the Greenlandic population, but rare or nonexistent in most others 63 , 64 , 65 , 66 . CREBRF has been shown to play a role in cellular energy storage and use, and may be implicated in cellular and organismal adaptation to nutritional stress 65 . ADCY3 colocalizes with MC4R at the primary cilia of a subset of hypothalamic neurons that have been implicated in body-weight regulation 67 .
GWAS have typically focused on biallelic, common genetic variation (MAF >5%), but have also been used to screen for the role of copy number variants (CNVs) in obesity. So far, only a few CNVs have been identified that have a convincing association with BMI, such as the 1p31.1 45-kb deletion near NEGR1 (ref. 68 ), which encodes a cell-adhesion molecule expressed in the brain 69 ; the 16p12.3 21-kb deletion upstream of GPRC5B 70 , which may modulate insulin secretion 71 ; the 10q11.22 CNV in PPYR1 (also known as NPY4R ) 72 , which encodes a potent anti-obesity agent known to inhibit food intake 73 ; and the 1p21.1 multi-allele CNV encompassing AMY1A 74 , which produces salivary α-amylase, a key enzyme in starch digestion 75 .
To determine the role of other types of variation in obesity, alternative genome-wide screens have been performed. For example, the impact of low-frequency and rare protein-coding variants has been tested using exome sequencing and exome array data 76 , 77 , 78 , 79 . It was speculated that low-frequency (MAF 1–5%) and rare (MAF <1%) variants would have larger effects than common variants, and thus be easier to detect. Nevertheless, even large-scale studies identified only a few robust associations for rare coding variants. For example, exome-wide screening based on array data from more than 400,000 individuals identified p.Tyr35Ter (rs13447324) in MC4R ; p.Arg190Gln (rs139215588) and p.Glu288Gly (rs143430880) in GIPR , which stimulates insulin secretion and mediates fat deposition 80 ; p.Arg95Ter (rs114285050) in GRP151 , which modulates habenular function that controls addiction vulnerability 81 ; and p.Arg769Ter (rs533623778) in PKHD1L1 , which has been involved in cancer development 77 , 78 . A recent study that leveraged WES data for more than 600,000 individuals identified 16 genes for which the burden of rare nonsynonymous variants was associated with BMI, including five brain-expressed G protein-coupled receptors ( CALCR , MC4R , GIPR , GPR151 and GPR75 ) 79 .
As obesity is a complex, multifactorial condition, some GWAS have integrated demographic factors (such as sex and age 82 ) and environmental factors (such as physical activity 83 , diet 84 or smoking 85 ) into their analyses. Despite sample sizes of more than 200,000 individuals, these genome-wide gene-by-environment (G×E) interaction analyses remain challenging and so far only 12 loci have been identified, the effects of which on obesity are attenuated or exacerbated by non-genetic factors. Nevertheless, the G×E interaction between the FTO locus and a healthy lifestyle has been robustly replicated. Specifically, increased physical activity or a healthy diet can attenuate the effect of the FTO locus on obesity risk by 30–40% 86 , 87 .
The increasing availability of large-scale cohorts and biobanks, such as the UK Biobank , the Million Veterans Project , All of Us , Biobank Japan and 23andMe , combined with ongoing work by the GIANT consortium, will boost sample sizes further to easily exceed 4 million participants in meta-analyses, expediting the discovery of many more obesity-associated loci. However, translation of GWAS-identified loci into new biological insights remains a major challenge.
From genes to biology
Despite the difficulties in validating causative mutations and variants, genetic studies into both rare and common obesity over the past two decades have revealed two surprisingly cogent, overarching biological messages: first, the leptin–melanocortin pathway is a key appetitive control circuit 31 , 88 (Fig. 4 ); and second, genes that are either enriched or exclusively expressed within the brain and CNS have a central role in obesity 89 .
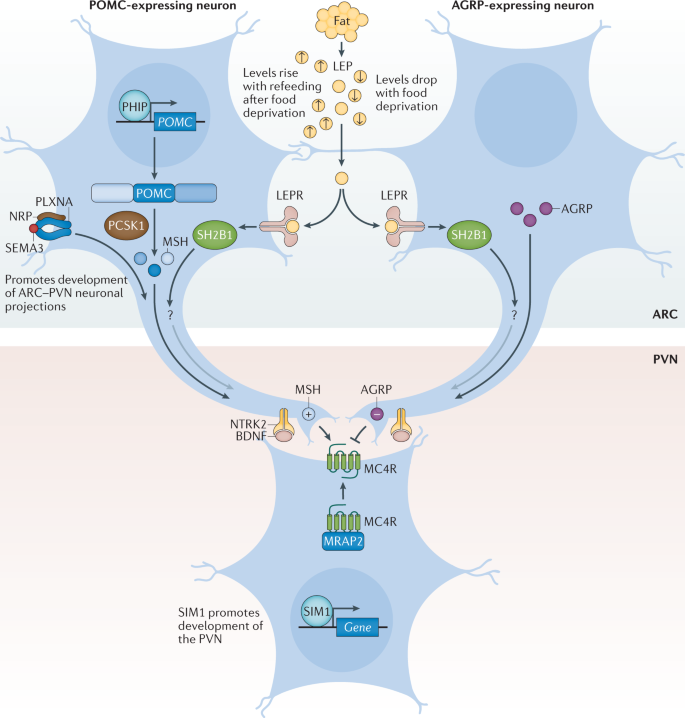
Pro-opiomelanocortin (POMC)-expressing neurons and agouti-related protein (AGRP)-expressing neurons within the arcuate nucleus of the hypothalamus (ARC) act to sense circulating leptin (LEP) levels, which reflect fat mass. These neurons signal to melanocortin 4 receptor (MC4R)-expressing neurons in the paraventricular nucleus of the hypothalamus (PVN), which controls appetite, thus linking long-term energy stores to feeding behaviour. Binding of class 3 semaphorins (SEMA3) to their receptors NRP and PLXNA influences the projection of POMC neurons to the PVN. Binding of brain-derived neurotrophic factor (BDNF) to its receptor neurotrophic receptor tyrosine kinase 2 (NTRK2) is thought to be an effector of leptin-mediated synaptic plasticity of neurons, including those in the ARC and PVN. The transcription factor SIM1 is crucial for the proper development of the PVN. +, agonist; −, antagonist; LEPR, leptin receptor; MRAP2, melanocortin receptor accessory protein 2; MSH, melanocyte-stimulating hormone; SH2B1, SH2B adaptor protein 1.
The leptin–melanocortin pathway and MC4R
Leptin is a key hormone secreted by adipocytes, which circulates at levels in proportion to fat mass 90 . Leptin also responds to acute changes in energy state, as its levels decrease with food deprivation and are restored during re-feeding. Administration of leptin to fasted mice abrogates many of the neuroendocrine consequences of starvation, suggesting that the normal biological role of leptin is to initiate the starvation response 91 . Leptin signals through the LEPR, which exists in several different isoforms. However, obesity-related effects of leptin are predominantly mediated by a long isoform that contains an intracellular domain (LEPRb), which is expressed in various regions of the CNS 90 .
Within the arcuate nucleus (ARC) of the hypothalamus, LEPRb is found on two populations of neurons at the heart of the melanocortin pathway, one of which expresses POMC and the other agouti-related protein (AGRP) 92 (Fig. 4 ). POMC is post-translationally processed by prohormone convertases to produce several biologically active moieties, including β-lipotrophin and β-endorphin, and, crucially, the melanocortin peptides adrenocorticotrophin (ACTH) and α-, β- and γ-melanocyte-stimulating hormone (MSH) 93 . The ARC POMC neurons project to MC4R neurons within the paraventricular nucleus (PVN) where melanocortin peptides signal to decrease food intake 92 . By contrast, AGRP acts as an endogenous antagonist of MC4R to increase food intake 92 , 94 . MC3R is another centrally expressed receptor that binds to both melanocortin peptides and AGRP; however, as mice with targeted deletions in the gene are not obese but instead have altered fat to lean mass ratio, MC3R is less likely to be related to food intake and more likely to be involved in nutrient partitioning 95 , 96 .
We can state with confidence that the fine balance of melanocortinergic agonism and AGRP antagonism of MC4R, in response to peripheral nutritional cues such as leptin, plays a central part in influencing appetitive drive 92 . The genetic evidence clearly supports this contention, with mutations in most genes of the melanocortin pathway resulting in hyperphagia and severe obesity in both humans and mice 31 , 88 . In fact, the vast majority of single-gene disruptions causing severe early-onset obesity in humans fall within this pathway, including LEPR , POMC , AGRP , MCR4R , PCSK1 (ref. 23 ), SH2B1 (ref. 97 ), PHIP 98 , MRAP2 (ref. 99 ) and SIM1 (ref. 100 ) (Fig. 4 ; Table 1 ). Mutations in MC4R in particular, are the most common single-gene defect leading to hyperphagia and obesity. Pathogenic mutations in MC4R are found in up to 5% of cases of severe childhood obesity 101 and up to 0.3% of the general population 101 , 102 . Of note, the degree of receptor dysfunction, as measured by in vitro assays, can predict the amount of food eaten at a test meal by an individual harbouring that particular mutation 101 . Thus MC4R does not act in a binary on/off manner, but as a rheostat; put simply, the melanocortin pathway is a ‘tunable’ system. In addition to regulating food intake, it also regulates food preference, with individuals who carry mutations in MC4R showing a preference for food with higher fat content 103 .
The importance of the melanocortin pathway in regulating feeding behaviour is highlighted by the identification of naturally occurring mutations in pathway genes in a wide range of different species where the appropriate selection pressure has been present (Table 1 ). For example, studies have found that 20–25% of Labrador retrievers, which are known to be more food-motivated than other dog breeds, carry a 14-bp deletion in POMC that disrupts the β-MSH and β-endorphin coding sequences and is associated with greater food motivation and increased body weight 104 . Also, certain breeds of pig have been shown to carry MC4R missense mutations that are associated with fatness, growth and food intake traits 105 . MC4R mutations even contribute to the adaptation and survival of blind Mexican cavefish to the nutrient-poor conditions of their ecosystem 106 .
Other neuronal circuits and molecules linked to severe obesity
It is now clear that in addition to engaging classical neuropeptide–receptor systems within the brain, leptin also rapidly modifies synaptic connections between neurons 107 , and that this structural plasticity is crucial to its downstream functions. One of the ways in which this plasticity is thought to be achieved is via brain-derived neurotrophic factor (BDNF) signalling to its receptor TrkB. BDNF is widely expressed in the CNS where it plays an important part in neuronal development 108 , 109 . In the hippocampus, BDNF contributes to synaptic plasticity and long-term potentiation associated with memory and learning 110 . However, evidence has emerged that implicates BDNF and TrkB in the regulation of mammalian eating behaviour and energy balance 111 . BDNF is downregulated by nutritional deprivation and upregulated by leptin within the ventromedial nucleus (VMN) of the hypothalamus 112 , although this regulation is probably indirect, as very few VMN BDNF neurons express the LEPR 113 (Fig. 4 ) and some evidence indicates that it acts at least in part downstream of melanocortin signalling 112 . Crucially, genetic disruption of BDNF 114 , 115 and TrkB 112 , 116 in both humans and mice results in hyperphagia and severe obesity.
Another group of neuronal proteins important in the development of neuronal circuitry and linked to energy balance are the class 3 semaphorins (SEMA3A–G). A study in humans found that 40 rare loss-of-function variants in SEMA3A–G and their receptors (PLXNA1–4, NRP1 and NRP2) were significantly enriched in 982 individuals with severe obesity compared with 4,449 controls 30 . Disruption of several of these genes in zebrafish caused increased somatic growth and/or adiposity, and experiments with mouse hypothalamic explants suggest that SEMA3 signalling via NRP2 receptors drives the development of POMC projections from the ARC to the PVN 30 . However, given that these results are from a single study, more data are required to confirm the exact role of class 3 semaphorins in energy homeostasis.
Insights from genetic loci linked to common obesity
Unlike candidate gene studies, GWAS make no a priori assumptions about the underlying biology that links genetic variants to a disease of interest. While this agnostic approach allows for new biological insights, the vast majority of GWAS-identified variants map to the non-coding parts of genes or to regions between genes. As such, they do not directly disrupt the protein-coding regions, but instead overlap with regulatory elements that influence expression of genes in close proximity or even over long distances.
However, even if the causative genes are unknown, pathway, tissue and functional enrichment analyses based on the genes located in the GWAS loci can provide insights into potential mechanisms. Since the very first GWAS for BMI 68 , 117 , such analyses have pointed to the CNS being a key player in body-weight regulation, consistent with insights from human and animal models of extreme obesity. Recent analyses that include the latest BMI-associated loci, combined with updated multi-omics databases and advanced computational tools, have further refined these observations. In addition to the hypothalamus and pituitary gland (which are both known appetite regulation sites), other brain areas have been highlighted, including the hippocampus and the limbic system (which are involved in learning, cognition and emotion) and the insula and the substantia nigra (which are related to addiction and reward) 58 , 89 , 118 , 119 . The enrichment of immune-related cells (such as lymphocytes and B cells) and adipose tissue was found to be weaker 58 .
Although enrichment analyses provide preliminary insights into the broad biology represented by genes in the GWAS loci, determining which genes, variants and/or underlying mechanisms are causal has proved an arduous task. For example, the FTO locus, which was identified more than a decade ago and harbours six genes, is the most extensively studied GWAS-identified obesity locus (Fig. 5 ). Despite its highly significant and widely replicated association with obesity 120 , the causal variants and/or genes in the FTO locus have not yet been pinpointed with convincing evidence, and the mechanisms by which the locus affects body weight have not been fully elucidated. Early functional follow-up analyses suggested that FTO itself might be responsible, as Fto deficiency in mice results in a lean phenotype, whereas Fto overexpression is associated with increased body weight 121 , 122 . Studies in mice have suggested that FTO plays a role in cellular nutrient sensing 123 , 124 . Other studies found evidence that FTO influences brain regions that affect appetite, reward processing and incentive motivation by regulating ghrelin levels in humans 125 or by controlling dopaminergic signalling in mice 126 , 127 . In addition, variants in the FTO locus were shown to alter a regulatory element that controls the transcription of Rpgrip1l in mice, a ciliary gene located immediately upstream of Fto 128 , 129 , 130 . Mice with reduced Rpgrip1l activity exhibit hyperphagic obesity, possibly mediated through diminished leptin signalling 128 , 129 , 130 . In recent years, studies in human and animal models have shown that variants in the FTO locus directly interact with the promoter of Irx3 , a gene located 0.5 Mb downstream of FTO . Irx3 -deficient mice were found to exhibit weight loss and increased metabolic rate with browning of white adipose tissue, without changes in physical activity or appetite 131 , 132 . Further in-depth functional characterization showed that rs1421085 in the FTO locus disrupts a conserved binding motif for the transcriptional repressor ARID5B, which leads to a doubling of IRX3 and IRX5 expression during early adipocyte differentiation 132 . The authors argue that increased expression of these genes results in a developmental shift from energy-dissipating beige adipocytes to energy-storing white adipocytes, a fivefold reduction in mitochondrial thermogenesis and increased lipid storage 132 . However, given that multiple studies have shown that the FTO locus is robustly associated with food intake, with no evidence to date linking it to changes in energy expenditure, the relevance of this observation to the actual observed human phenotype still needs to be explored 133 . A recent study reports that the FTO locus affects gene expression in multiple tissues, including adipose tissue and brain, and, more broadly, that the genetic architecture of disease-associated loci may involve extensive pleiotropy and allelic heterogeneity across tissues 134 .
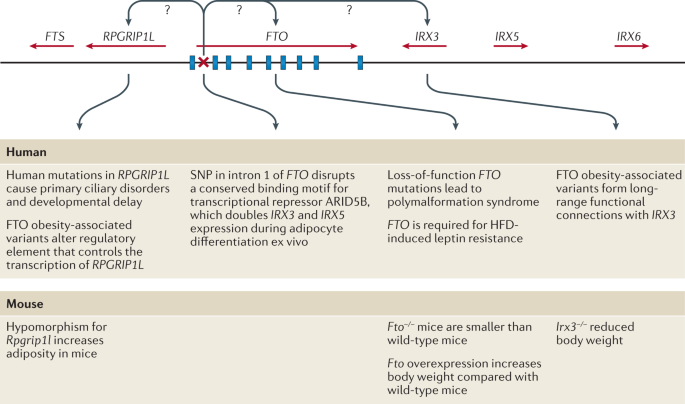
FTO contains nine exons (depicted by blue rectangles) and the body mass index (BMI)-associated SNP identified in genome-wide association studies (depicted by a red ×) maps to intron 1. IRX3 and RPGRIP1L have both been proposed to be the causal genes for obesity within the locus and to act on body weight through distinct mechanisms. HFD, high-fat diet.
Besides the FTO locus, functional follow-up analyses have been performed for only a few obesity-associated GWAS loci. For example, early studies identified a cluster of variants just downstream of TMEM18 (refs 68 , 117 ). TMEM18 encodes a poorly characterized transmembrane protein that is highly conserved across species and widely expressed across tissues, including in several regions of the brain 135 , 136 . Tmem18 deficiency in mice results in a higher body weight owing to increased food intake, whereas Tmem18 overexpression reduces food intake and limits weight gain 136 . A knockdown experiment in Drosophila melanogaster suggests that TMEM18 affects carbohydrate and lipid levels by disrupting insulin and glucagon signalling 137 .
Two other GWAS loci for which functional analyses have been performed are located just upstream of CADM1 (ref. 82 ) and in CADM2 (ref. 70 ), genes that encode cell-adhesion proteins of the immunoglobulin superfamily and mediate synaptic assembly in the CNS 138 . The BMI-increasing alleles at each locus are associated with increased expression of CADM1 and CADM2 in the hypothalamus 139 , 140 . Deficiency of either Cadm1 or Cadm2 in mice results in a lower body weight and increased insulin sensitivity, glucose tolerance and energy expenditure without any change in food intake 139 , 140 . Conversely, increased neuronal expression of either Cadm1 or Cadm2 is associated with elevated body weight 139 , 140 . Furthermore, CADM1 is expressed in POMC neurons and Cadm1 deficiency leads to an increase in the number of excitatory synapses, suggestive of an increased synaptic plasticity 140 . Cadm2 -deficient mice exhibit increased locomotor activity and higher core body temperature 139 .
Another GWAS locus, just upstream of NEGR1 , harbours two deletions associated with increased obesity risk 68 , 117 , 141 . These deletions do not overlap with the coding sequence of NEGR1 , but encompass a conserved transcription factor-binding site for NKX6.1 , a potent transcriptional repressor 68 , 141 . Loss of binding of NKX6.1 leads to higher NEGR1 expression 141 , which is consistent with the observation that BMI-increasing alleles (that is, deletions) at this locus are associated with higher NEGR1 expression in the brain. Similar to CADM1 and CADM2, NEGR1 is a cell-adhesion molecule of the immunoglobulin superfamily that is expressed in several regions of the brain and has been shown to have a role in brain connectivity 69 , 142 , a process believed to be important in obesity 143 . NEGR1 deficiency in mice was shown to result in lower body weight, mainly due to reduced lean mass, mediated by lower food intake 144 . However, two other functional studies, one in mice and one in rats, found that knockdown of Negr1 expression resulted in the opposite phenotype — increased body weight and food intake 145 , 146 . While NEGR1 deficiency in mice was found to impair core behaviours, so far, findings and proposed mechanisms are not fully aligned 69 , 147 , 148 , 149 .
Taken together, functional follow-up analyses for these loci are slowly expanding our understanding of the pathophysiology that drives weight gain. However, many more obesity-associated loci are waiting to be translated into new biological insights. A major hurdle in translating GWAS loci into plausible candidate genes and appropriate paradigms for functional research is the annotation of the associated variants in a locus. Defining the regulatory function of the non-coding variants, identifying their putative effector transcripts and determining their tissues of action remains an ongoing challenge. The advent of high-throughput genome-scale technologies for mapping regulatory elements, combined with comprehensive multi-omics databases, advanced computational tools and the latest genetic engineering and molecular phenotyping approaches, is poised to speed up the translation of GWAS loci into meaningful biology 150 .
Converging results from monogenic and polygenic forms of obesity
Gene discovery is often dichotomized by allele frequency and disease prevalence; that is, mutations are sought for monogenic forms of obesity and common variants for polygenic obesity (Fig. 2 ). However, it is increasingly recognized that monogenic and polygenic forms of obesity are not discrete entities. Instead, they lie on a spectrum and share — at least in part — the same biology. As GWAS have continued to discover more obesity-associated loci, an increasing number of these loci harbour genes that were first identified for extreme and early-onset obesity in humans or animal models, including MC4R 151 , 152 , BDNF 117 , SH2B1 (refs 68 , 117 ), POMC 70 , LEP 51 , 153 , LEPR 52 , 154 , NPY 155 , SIM1 (ref. 155 ), NTRK2 (ref. 58 ), PCSK1 (ref. 154 ) and KSR2 (ref. 77 ). In fact, most of these genes encode components of the leptin–melanocortin and BDNF–TrkB signalling pathways (Table 1 ). Thus, whereas genetic disruption of components of these pathways results in severe obesity, genetic variants in or near these same genes that have more subtle effects on their expression will influence where an individual might sit in the normal distribution of BMI.
Although most genes have been first identified for extreme forms of obesity, a locus harbouring ADCY3 was first identified in GWAS for common obesity 77 , and ADCY3 was subsequently confirmed as having a role in extreme obesity 63 , 64 . ADCY3 encodes an adenylate cyclase that catalyses the synthesis of cAMP, an important second messenger in signalling pathways. There is some evidence that ADCY3 (adenylate cyclase) colocalizes with MC4R at the primary cilia of PVN neurons 67 and that cilia are required specifically on MC4R-expressing neurons for the control of energy homeostasis 156 . In mice, disruption of Adcy3 or Mc4r in the cilia of these neurons impairs melanocortin signalling, resulting in hyperphagia and obesity 67 .
As more GWAS loci are reported, we expect that findings across different lines of obesity research will continue to converge, providing accumulating evidence for new biology.
From genes to clinical care
Genetic insights from gene discovery efforts are increasingly being used in the context of precision medicine in ways that directly affect health. Knowing a patient’s genotype may enable a more precise diagnosis of the type of obesity, which in turn allows the prescription of personalized treatment or prevention strategies. Furthermore, knowing an individual’s genetic susceptibility to obesity early in life may help to more accurately predict those most at risk of gaining weight in the future.

Use of genotype information in treatment of obesity
When a disease is caused by a single mutation and the environmental contribution is limited, as is the case for some forms of extreme and early-onset obesity, a genetic test can be instrumental in correctly diagnosing patients. Although no standard genetic testing panel is currently available for extreme and early-onset obesity, some clinics, research centres and pharmaceutical companies sequence well-known candidate genes to identify the functional mutation that may be the cause of a patient’s excess body weight. Such a genetic diagnosis can lessen the feelings of guilt and blame for the patient, and alleviate social stigma and discrimination. Importantly, a genetic diagnosis can inform disease prognosis and, in some cases, it will determine treatment. To date, there are two treatments for obesity that are tailored to patient genotype.
The prototype of genotype-informed treatment for obesity is the administration of recombinant human leptin in patients who are leptin-deficient owing to mutations in the LEP gene 157 , 158 . Although congenital leptin deficiency is exceptionally rare (only 63 cases have been reported to date 28 ), leptin replacement therapy has been remarkably beneficial for these patients by substantially reducing food intake, body weight and fat mass, and normalizing endocrine function 157 , 158 . It has literally transformed their lives.
The second genotype-informed treatment for obesity is setmelanotide, a selective MC4R agonist that was recently approved by the FDA for rare monogenic obesity conditions including LEPR, PCSK1 and POMC deficiency 159 . Setmelanotide acts as a substitute for the absent MSH in patients with POMC deficiency owing to mutations in POMC or PCSK1 , and in patients with LEPR deficiency owing to mutations in LEPR , which is essential for POMC function 160 , 161 , 162 . Daily subcutaneous injection of setmelanotide results in substantial weight loss and in reduction of hunger 160 , 161 , 162 . After a 1-year treatment with setmelanotide in phase III trials, patients with POMC deficiency lost on average 25.6% of their initial weight, with 80% of patients achieving at least a 10% weight loss 162 . The adverse effects of setmelanotide treatment are minor, and include hyperpigmentation, nausea and/or vomiting, penile erection and injection site reactions. Weight loss in patients with LEPR deficiency was less pronounced; on average, they lost 12.5% of their initial weight, with only 45% of patients achieving at least a 10% weight loss 162 . The difference in weight loss between the two patient groups may be because POMC deficiency directly affects the production of MC4R ligands (α-MSH and β-MSH), whereas LEPR deficiency affects signalling upstream of POMC 162 . As such, setmelanotide may be able to completely restore MC4R signalling in POMC deficiency, but only partially in LEPR deficiency. Even though the average weight loss in POMC-deficient patients was twice that in LEPR-deficient patients, the reduction in hunger was substantially larger in LEPR-deficient patients (−43.7%) than in POMC-deficient patients (−27.1%) 162 . The reasons for the discrepancy between weight loss and reduction in hunger remain to be studied in greater depth. It has been estimated that in the USA, >12,800 individuals carry mutations in the melanocortin pathway for whom setmelanotide may be more effective for weight loss than any other treatment 163 . Although 12,800 carriers represent only a fraction (0.004%) of the adult population in the USA, and not all of these mutation carriers are overweight or obese, for the patients for whom setmelanotide is effective, it may end a lifelong battle to lose weight 163 . In patients without genetic defects, neither setmelanotide nor leptin administration have, to date, demonstrated a substantial effect on weight loss 164 , 165 .
These two genotype-informed treatments show how insight into the underlying biological mechanisms can guide the development of molecules and medications that restore impaired pathways, at least in monogenic forms of obesity caused by deficiency of one protein. Nevertheless, there remain substantial obstacles in the transition from conventional to precision medicine for monogenic obesity, which would require the adoption of systematic WES for individuals suspected to be carriers of deleterious mutations, and eventually even standardized screening at birth. We are clearly a long way from such a scenario at present.
Use of genotype information in prediction of obesity
As more variants are being discovered for common obesity, there is a growing expectation that genetic information will soon be used to identify individuals at risk of obesity. Knowing a person’s genetic susceptibility would allow for a more accurate prediction of who is at risk of gaining weight and give an opportunity to intervene earlier to prevent obesity more effectively. Genetic susceptibility to complex disease, including obesity, is assessed using a polygenic score (PGS). PGSs to assess obesity susceptibility are based on GWAS for BMI (PGS BMI ), the latest of which includes data on more than 2 million variants and explains 8.4% of the variation in BMI 166 . The average BMI of individuals with a high PGS BMI (top decile) is 2.9 kg m −2 (equivalent to 8 kg in body weight) higher and their odds of severe obesity (BMI ≥40 kg m −2 ) is 4.2-fold higher than those with a lower PGS BMI (lowest nine deciles) 166 .
Despite these strong associations with BMI and obesity, the predictive performance of the PGS BMI is weak, which is unsurprising given its limited explained variance. For example, using the same PGS BMI and data from the UK Biobank, we estimate that the area under the receiver operating characteristic curve (AUC ROC ) is only 0.64 to predict obesity. This means that the probability that an individual with obesity has a higher PGS BMI than an individual without obesity is 0.64. However, for a PGS to have clinical utility, the AUC ROC needs to be much higher (>0.80). In addition, we calculated the extent to which a PGS BMI ≥90th percentile correctly classifies individuals with obesity (Fig. 6 ). We found that such a predictive test (PGS BMI ≥90th percentile) has a positive predictive value of 0.43, meaning that of those who were predicted to develop obesity, only 43% actually developed obesity. Its sensitivity is 0.19, which means that of the individuals who developed obesity, only 19% had been correctly classified by the PGS BMI . Given that the current treatment options for obesity are low risk, or even generally beneficial, the high false-positive rate is less concerning than the low sensitivity, as some at-risk individuals may miss the opportunity for early prevention.
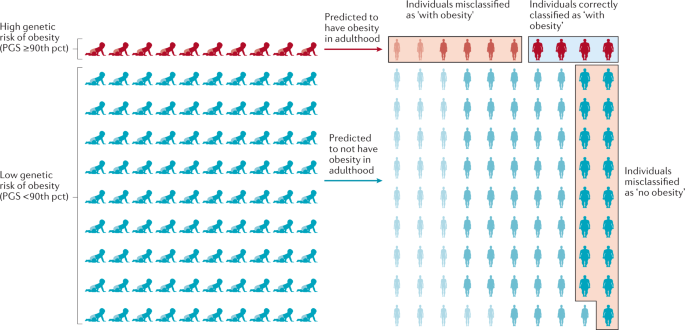
The outcome is illustrated for a polygenic score (PGS) that assumes that individuals with a score in the highest decile (≥90th percentile (pct)) will develop obesity, has a positive predictive value of 0.4 and a sensitivity of 0.19. Of ten individuals with a high score classified by the PGS as ‘with obesity’, four will be classified correctly but the other six will be misclassified and will not develop obesity — a positive predictive value of 0.4. Likewise, 17 of the 90 individuals with a score <90th pct who are predicted to not develop obesity, will develop obesity. Thus, only four of the 21 individuals who developed obesity were correctly classified by the PGS — a sensitivity of 0.19. Misclassified individuals are indicated by the red boxes, individuals correctly classified as ‘with obesity’ are indicated by a blue box. Adapted with permission from ref. 170 , Elsevier.
Thus, the current PGS BMI has a high rate of misclassification and does not reliably predict who is at risk of developing obesity and who is not. The predictive ability of PGSs are expected to improve as GWAS increase in sample size and algorithms to calculate the scores become more refined. Nevertheless, given the importance of socio-demographic, lifestyle and clinical risk factors in the aetiology of obesity, it is unlikely that a PGS BMI will ever be able to accurately predict obesity on its own. Instead, effective prediction models will have to include genetic and non-genetic factors, including a broad spectrum of demographic, environmental, clinical and possibly molecular markers, as well.
Conclusions and future perspectives
What initially began as two apparently distinct approaches, one studying rare Mendelian causes of extreme obesity, and the other exploring complex polygenic influences of population body-weight distribution, have eventually converged on the central role of the brain in regulating body weight. In particular, both approaches have highlighted the roles of the leptin–melanocortin pathway and TrkB–BDNF signalling. Perhaps it seems obvious now, but it was by no means certain that, just because genetic disruption of a pathway resulted in a severe phenotype, polymorphisms within that same pathway would produce a more subtle and nuanced result.
The GWAS approach is hypothesis-free, with the promise to reveal new genes that point to new biology and pathways. However, for the vast majority of the >1,000 GWAS-identified loci, we do not know which genes are causal, what cells, tissues and organs they act in to affect body weight, and we do not understand the underlying mechanisms. The translation from variant to function is a well-known challenge 167 , but with increasing availability of new omics data, high-throughput technologies and advanced analytical approaches, there is an unprecedented opportunity to speed up the translation of hundreds of GWAS loci.
Sample size remains a major driver for gene discovery. In an ongoing collaboration that combines data from more than 3 million individuals of diverse ancestry from the GIANT consortium, the UK Biobank and 23andMe, the number of BMI-associated GWAS loci is set to double. Also, a recent WES effort of more than 640,000 individuals has demonstrated that rare mutations are discoverable when sample sizes are sufficiently large 79 . However, alternative study designs, a focus on more refined phenotypes or a focus on population subgroups (that is, more homogeneous groups of individuals with similar outcomes) could further add to gene discovery.
Translation of only a few dozen of the GWAS-identified loci could tremendously improve our insights into the biology of obesity and possibly reveal new therapeutic targets. It would also take us a little closer to the ‘holy grail’ — the ability to move away from a failed ‘one-size-fits-all’ strategy, and towards true precision medicine for obesity, metabolic disease and other diet-related illnesses.
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Article Google Scholar
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282 , 1523–1529 (1999).
Article CAS PubMed Google Scholar
Fontaine, K. R. & Barofsky, I. Obesity and health-related quality of life. Obes. Rev. 2 , 173–182 (2001).
Bhattacharya, I., Ghayor, C., Perez Dominguez, A. & Weber, F. E. From influenza virus to novel corona virus (SARS-CoV-2)—the contribution of obesity. Front. Endocrinol. 11 , 556962 (2020).
Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 369 , m1966 (2020).
Article PubMed PubMed Central Google Scholar
Cummings, M. J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395 , 1763–1770 (2020).
Article CAS PubMed PubMed Central Google Scholar
Zhao, X. et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front. Endocrinol. 11 , 595109 (2020).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390 , 2627–2642 (2017).
Maes, H. H., Neale, M. C. & Eaves, L. J. Genetic and environmental factors in relative body weight and human obesity. Behav. Genet. 27 , 325–351 (1997).
Elks, C. E. et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. 3 , 29 (2012). This paper reports a large-scale meta-analysis of heritability data of twin and family studies .
Chami, N., Preuss, M., Walker, R. W., Moscati, A. & Loos, R. J. F. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 17 , e1003196 (2020). This study shows that the effect of MC4R mutations on BMI and obesity risk is attenuated by the polygenic background .
Kaur, Y., de Souza, R. J., Gibson, W. T. & Meyre, D. A systematic review of genetic syndromes with obesity. Obes. Rev. 18 , 603–634 (2017).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41 , 317–318 (1950).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153 , 1127–1128 (1966).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372 , 425–432 (1994). This paper reports the cloning of leptin, which provided the first molecular evidence that feeding and regulation of body weight had a hormonal basis .
Article CAS Google Scholar
Chen, H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db / db mice. Cell 84 , 491–495 (1996). This paper reports the cloning of the LEPR and the localization of its signalling version, which highlights the role of the brain in the control of appetitive behaviour .
Bultman, S. J., Michaud, E. J. & Woychik, R. P. Molecular characterization of the mouse agouti locus. Cell 71 , 1195–1204 (1992).
Miller, M. W. et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 7 , 454–467 (1993).
Lu, D. et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371 , 799–802 (1994).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385 , 165–168 (1997).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 , 903–908 (1997). This work demonstrates that leptin signalling is also relevant in the human context, and provides early evidence, together with mutations in PCSK1 , of a monogenic cause of severe human obesity .
Clement, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 , 398–401 (1998). This work confirms the observations from mice about leptin signalling to the brain .
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16 , 303–306 (1997). This study, together with mutations in the leptin gene, provides early evidence of a monogenic cause of severe human obesity, and also highlights the role of the nascent melanocortin pathway in body-weight regulation .
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88 , 131–141 (1997).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20 , 111–112 (1998).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in MC4R is associated with a dominant form of obesity. Nat. Genet. 20 , 113–114 (1998). This paper, together with Yeo et al. (1998), shows that heterozygous mutations in MC4R result in severe human obesity, establishing the role of the central melanocortin pathway in regulating human appetitive behaviour .
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19 , 155–157 (1998). This paper provides direct evidence that melanocortin peptides play a key role in the regulation of energy homeostasis .
Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5 , 1066–1070 (1999).
Challis, B. G. et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc. Natl Acad. Sci. USA 101 , 4695–4700 (2004).
van der Klaauw, A. A. et al. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell 176 , 729–742.e18 (2019).
Farooqi, S. & O’Rahilly, S. Genetics of obesity in humans. Endocr. Rev. 27 , 710–718 (2006).
Saeed, S., Arslan, M. & Froguel, P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity 26 , 474–484 (2018).
Article PubMed Google Scholar
Saeed, S. et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity 23 , 1687–1695 (2015).
Saeed, S. et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 69 , 1424–1438 (2020).
Kurokawa, N. et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44 833 individuals. Int. J. Obes. 32 , 1240–1249 (2008).
Shugart, Y. Y. et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 17 , 1050–1055 (2009).
Benzinou, M. et al. Endocannabinoid receptor 1 gene variations increase risk for obesity and modulate body mass index in European populations. Hum. Mol. Genet. 17 , 1916–1921 (2008).
Wang, D. et al. Association of the MC4R V103I polymorphism with obesity: a Chinese case–control study and meta-analysis in 55,195 individuals. Obesity 18 , 573–579 (2010).
Nead, K. T. et al. Contribution of common non-synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta-analysis with evidence from up to 331 175 individuals. Hum. Mol. Genet. 24 , 3582–3594 (2015).
Tonjes, A., Scholz, M., Loeffler, M. & Stumvoll, M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 29 , 2489–2497 (2006).
Rankinen, T. et al. The human obesity gene map: the 2005 update. Obesity 14 , 529–644 (2006).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 , 889–894 (2007). This paper describes the first GWAS (for type 2 diabetes) to identify a locus ( FTO ) robustly associated with BMI .
Scuteri, A. et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3 , e115 (2007).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47 , D1005–D1012 (2019).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700 000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Javed, A. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr. Obes. 10 , 234–244 (2015).
Kilpelainen, T. O. et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 43 , 753–760 (2011).
Lu, Y. et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 7 , 10495 (2016).
Zillikens, M. C. et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat. Commun. 8 , 80 (2017).
Chu, A. Y. et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 49 , 125–130 (2017).
Kilpelainen, T. O. et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 7 , 10494 (2016).
Sun, Q. et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum. Mol. Genet. 19 , 1846–1855 (2010).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15 , e1007603 (2019).
Orthofer, M. et al. Identification of ALK in thinness. Cell 181 , 1246–1262.e22 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019).
Felix, J. F. et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25 , 389–403 (2016).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Akiyama, M. et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49 , 1458–1467 (2017).
Ng, M. C. Y. et al. Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet. 13 , e1006719 (2017).
Gurdasani, D. et al. Uganda genome resource enables insights into population history and genomic discovery in Africa. Cell 179 , 984–1002.e36 (2019).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570 , 514–518 (2019).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51 , 584–591 (2019).
Grarup, N. et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 50 , 172–174 (2018).
Saeed, S. et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat. Genet. 50 , 175–179 (2018).
Minster, R. L. et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat. Genet. 48 , 1049–1054 (2016).
Andersen, M. K. et al. The derived allele of a novel intergenic variant at chromosome 11 associates with lower body mass index and a favorable metabolic phenotype in Greenlanders. PLoS Genet. 16 , e1008544 (2020).
Siljee, J. E. et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet. 50 , 180–185 (2018).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41 , 25–34 (2009).
Singh, K. et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 9 , 5457 (2019).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42 , 937–948 (2010).
Soni, A., Amisten, S., Rorsman, P. & Salehi, A. GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem. Biophys. Res. Commun. 441 , 643–648 (2013).
Jarick, I. et al. Novel common copy number variation for early onset extreme obesity on chromosome 11q11 identified by a genome-wide analysis. Hum. Mol. Genet. 20 , 840–852 (2011).
Sainsbury, A. et al. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol. Cell Biol. 23 , 5225–5233 (2003).
Falchi, M. et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat. Genet. 46 , 492–497 (2014).
Meisler, M. H. & Ting, C. N. The remarkable evolutionary history of the human amylase genes. Crit. Rev. Oral Biol. Med. 4 , 503–509 (1993).
Hendricks, A. E. et al. Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci. Rep. 7 , 4394 (2017).
Turcot, V. et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50 , 26–41 (2018). This large-scale exome-wide discovery study reports the use of array data to identify rare coding variations associated with BMI .
Emdin, C. A. et al. Analysis of predicted loss-of-function variants in UK Biobank identifies variants protective for disease. Nat. Commun. 9 , 1613 (2018).
Akbari, P. et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 373 , eabf8683 (2021). This large-scale exome-wide discovery study reports the use of WES data to identify mutations associated with BMI .
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132 , 2131–2157 (2007).
Antolin-Fontes, B. et al. The habenular G-protein-coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc. Natl Acad. Sci. USA 117 , 5502–5509 (2020).
Winkler, T. W. et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 11 , e1005378 (2015).
Graff, M. et al. Genome-wide physical activity interactions in adiposity — a meta-analysis of 200,452 adults. PLoS Genet. 13 , e1006528 (2017).
Smith, C. E. et al. Genome-wide interactions with dairy intake for body mass index in adults of European descent. Mol. Nutr. Food Res. 62 , 1700347 (2018).
Justice, A. E. et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 8 , 14977 (2017).
Qi, Q. et al. Fried food consumption, genetic risk, and body mass index: gene–diet interaction analysis in three US cohort studies. BMJ 348 , g1610 (2014).
Kilpelainen, T. O. et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 8 , e1001116 (2011).
Yeo, G. S. H. Genetics of obesity: can an old dog teach us new tricks? Diabetologia 60 , 778–783 (2017).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015). This large-scale GWAS for BMI shows that BMI-associated loci frequently localize in or near genes that act in the brain .
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395 , 763–770 (1998).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382 , 250–252 (1996).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24 , 155–163 (1999).
Bertagna, X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 23 , 467–485 (1994).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278 , 135–138 (1997).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141 , 3518–3521 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 26 , 97–102 (2000).
Doche, M. E. et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122 , 4732–4736 (2012).
Marenne, G. et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cell Metab. 31 , 1107–1119.e12 (2020).
Asai, M. et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341 , 275–278 (2013).
Michaud, J. L. et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10 , 1465–1473 (2001).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348 , 1085–1095 (2003).
Wade, K. H. et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat. Med. 27 , 1088–1096 (2021). This paper establishes the frequency of loss-of-function mutations in MC4R to be 0.3%, much more common than previously appreciated .
van der Klaauw, A. et al. Role of melanocortin signalling in the preference for dietary macronutrients in human beings. Lancet 385 , S12 (2015).
Raffan, E. et al. A deletion in the canine POMC gene is associated with weight and appetite in obesity-prone Labrador retriever dogs. Cell Metab. 23 , 893–900 (2016).
Kim, K. S., Larsen, N., Short, T., Plastow, G. & Rothschild, M. F. A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm. Genome 11 , 131–135 (2000).
Aspiras, A. C., Rohner, N., Martineau, B., Borowsky, R. L. & Tabin, C. J. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proc. Natl Acad. Sci. USA 112 , 9668–9673 (2015).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304 , 110–115 (2004).
Davies, A. M. The role of neurotrophins in the developing nervous system. J. Neurobiol. 25 , 1334–1348 (1994).
McAllister, A. K., Katz, L. C. & Lo, D. C. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18 , 767–778 (1997).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92 , 8856–8860 (1995).
Kernie, S. G., Liebl, D. J. & Parada, L. F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 19 , 1290–1300 (2000).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6 , 736–742 (2003).
Xu, B. & Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 17 , 282–292 (2016).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15 , 1748–1757 (2001).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55 , 3366–3371 (2006).
Yeo, G. S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7 , 1187–1189 (2004).
Thorleifsson, G. et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 41 , 18–24 (2009).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50 , 621–629 (2018).
Ndiaye, F. K. et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int. J. Obes. 44 , 539–543 (2020).
Loos, R. J. & Yeo, G. S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10 , 51–61 (2014).
Church, C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 42 , 1086–1092 (2010).
Fischer, J. et al. Inactivation of the Fto gene protects from obesity. Nature 458 , 894–898 (2009).
Cheung, M. K., Gulati, P., O’Rahilly, S. & Yeo, G. S. FTO expression is regulated by availability of essential amino acids. Int. J. Obes. 37 , 744–747 (2013).
Gulati, P. et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl Acad. Sci. USA 110 , 2557–2562 (2013).
Karra, E. et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 123 , 3539–3551 (2013).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16 , 1042–1048 (2013).
Ruud, J. et al. The fat mass and obesity-associated protein (FTO) regulates locomotor responses to novelty via D2R medium spiny neurons. Cell Rep. 27 , 3182–3198.e9 (2019).
Stratigopoulos, G., LeDuc, C. A., Cremona, M. L., Chung, W. K. & Leibel, R. L. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J. Biol. Chem. 286 , 2155–2170 (2011).
Stratigopoulos, G. et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 19 , 767–779 (2014).
Stratigopoulos, G. et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126 , 1897–1910 (2016).
Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507 , 371–375 (2014).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373 , 895–907 (2015).
O’Rahilly, S., Coll, A. P. & Yeo, G. S. FTO obesity variant and adipocyte browning in humans. N. Engl. J. Med. 374 , 191 (2016).
PubMed Google Scholar
Sobreira, D. R. et al. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science 372 , 1085–1091 (2021).
Almen, M. S. et al. The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. BMC Med. Genet. 11 , 58 (2010).
Larder, R. et al. Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc. Natl Acad. Sci. USA 114 , 9421–9426 (2017). Aside from the efforts at characterizing the FTO locus, this paper establishes a plausible role for yet another GWAS-identified candidate gene, TMEM18 , in energy homeostasis .
Wiemerslage, L. et al. The Drosophila ortholog of TMEM18 regulates insulin and glucagon-like signaling. J. Endocrinol. 229 , 233–243 (2016).
Biederer, T. et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297 , 1525–1531 (2002).
Yan, X. et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol. Metab. 8 , 180–188 (2018).
Rathjen, T. et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 20 , 1096–1103 (2017).
Wheeler, E. et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat. Genet. 45 , 513–517 (2013).
Schafer, M., Brauer, A. U., Savaskan, N. E., Rathjen, F. G. & Brummendorf, T. Neurotractin/kilon promotes neurite outgrowth and is expressed on reactive astrocytes after entorhinal cortex lesion. Mol. Cell. Neurosci. 29 , 580–590 (2005).
Matikainen-Ankney, B. A. & Kravitz, A. V. Persistent effects of obesity: a neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 1428 , 221–239 (2018).
Lee, A. W. et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE 7 , e41537 (2012).
Joo, Y., Kim, H., Lee, S. & Lee, S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int. J. Obes. 43 , 1769–1782 (2019).
Boender, A. J., van Gestel, M. A., Garner, K. M., Luijendijk, M. C. & Adan, R. A. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiol. Rep. 2 , e12083 (2014).
Singh, K. et al. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease-associated negr1 gene. Front. Mol. Neurosci. 11 , 30 (2018).
Szczurkowska, J. et al. NEGR1 and FGFR2 cooperatively regulate cortical development and core behaviours related to autism disorders in mice. Brain 141 , 2772–2794 (2018).
PubMed PubMed Central Google Scholar
Noh, K. et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 24 , 1189–1205 (2019).
Cano-Gamez, E. & Trynka, G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 11 , 424 (2020).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40 , 768–775 (2008).
Chambers, J. C. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat. Genet. 40 , 716–718 (2008).
Yaghootkar, H. et al. Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes 69 , 2806–2818 (2020).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104 , 65–75 (2019).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28 , 166–174 (2019).
Wang, Y. et al. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Invest. 131 , e142064 (2021).
Article CAS PubMed Central Google Scholar
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341 , 879–884 (1999). This paper is the first description of the treatment of leptin deficiency using leptin replacement therapy .
Farooqi, I. S. & O’Rahilly, S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 223 , T63–T70 (2014).
Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 48 , 101206 (2021).
Kuhnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016). This paper is the first to demonstrate that the melanocortin pathway is a relevant and, so far, safe therapeutic target for the treatment of at least rare genetic causes of obesity .
Clement, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24 , 551–555 (2018).
Clement, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Ayers, K. L. et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J. Clin. Endocrinol. Metab. 103 , 2601–2612 (2018).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282 , 1568–1575 (1999).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 6 , 1321–1329 (2017).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177 , 587–596.e9 (2019). This study describes a comprehensive PGS and its association with BMI throughout the life course .
Loos, R. J. F. 15 years of genome-wide association studies and no signs of slowing down. Nat. Commun. 11 , 5900 (2020).
Pearce, L. R. et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell 155 , 765–777 (2013).
Greenfield, J. R. et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 360 , 44–52 (2009).
Torkamani, A. & Topol, E. Polygenic risk scores expand to obesity. Cell 177 , 518–520 (2019).
Download references
Acknowledgements
R.J.F.L. is supported by funding from Novo Nordisk Foundation (NNF Laureate Award) and the US National Institutes of Health (R01DK110113; R01DK107786; R01HL142302; R01 DK124097). G.S.H.Y. is supported by the Medical Research Council (MRC Metabolic Diseases Unit (MC_UU_00014/1)). The authors thank M. Guindo Martinez for her help with creating data for Fig. 3 and Supplementary Tables 1 and 2.
Author information
Authors and affiliations.
Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark
Ruth J. F. Loos
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Mindich Child Health and Development Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA
MRC Metabolic Diseases Unit, University of Cambridge Metabolic Research Laboratories, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK
Giles S. H. Yeo
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Correspondence to Ruth J. F. Loos or Giles S. H. Yeo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Genetics thanks D. Meyre, T. Fall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
23andMe: https://research.23andme.com/collaborate/
All of Us: https://allofus.nih.gov/
Biobank Japan: http://jenger.riken.jp/en/
Genetic Investigation for ANthropometric Traits (GIANT) consortium: https://portals.broadinstitute.org/collaboration/giant/index.php/Main_Page
Million Veterans Project: https://www.research.va.gov/mvp/
NCD Risk Factor Collaboration (NCD RisC): https://www.ncdrisc.org/data-visualisations.html
UK Biobank: https://www.ukbiobank.ac.uk/
Supplementary information
Supplementary information.
An environment that promotes weight gain.
A severe, early-onset form of obesity, caused by a single-gene mutation, with little or no influence of the environment.
A common multifactorial form of obesity, resulting from an interaction between the obesogenic environment and hundreds of genetic variants.
An approach used to understand the function of a gene by analysing the consequences of genetically manipulating specific sequences within the gene.
A hypothesis-driven approach to study the effect of a given gene (chosen based on the current understanding of its biology and pathophysiology) on susceptibility to the phenotype under study.
A method that relies on the relatedness of study participants to test whether certain chromosomal regions co-segregate with a disease or trait across generations.
(GWAS). A hypothesis-generating approach that screens whole genomes for associations between genetic variants and a phenotype of interest at much higher resolution than is possible for genome-wide linkage studies, and is thus better able to narrow down the associated locus.
(PGS). A measure used to assess an individual’s genetic susceptibility to disease, calculated by summing the number of disease-increasing alleles, weighted by each variant’s effect size observed in a genome-wide association study.
(AUC ROC ). A metric used to assess the ability of a predictor to discriminate between individuals with and without a disease. The AUC ranges from 0.50 (equal to tossing a coin) to 1.0 (perfect prediction).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Loos, R.J.F., Yeo, G.S.H. The genetics of obesity: from discovery to biology. Nat Rev Genet 23 , 120–133 (2022). https://doi.org/10.1038/s41576-021-00414-z
Download citation
Accepted : 17 August 2021
Published : 23 September 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41576-021-00414-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic effects of sequence-conserved enhancer-like elements on human complex traits.
- Wing Hung Wong
Genome Biology (2024)
A low-calorie meal replacement improves body composition and metabolic parameters in shift workers with overweight and obesity: a randomized, controlled, parallel group trial
- Piumika Sooriyaarachchi
- Ranil Jayawardena
- Neil A. King
Nutrition & Metabolism (2024)
Hyperphagia and impulsivity: use of self-administered Dykens’ and in-house impulsivity questionnaires to characterize eating behaviors in children with severe and early-onset obesity
- Lara Arnouk
- Hélène Chantereau
- Béatrice Dubern
Orphanet Journal of Rare Diseases (2024)
Associations between maternal pre-pregnancy BMI and infant striatal mean diffusivity
- Aylin Rosberg
- Harri Merisaari
- Jetro J. Tuulari
BMC Medicine (2024)
Identifying latent genetic interactions in genome-wide association studies using multiple traits
- Andrew J. Bass
- Shijia Bian
- Michael P. Epstein
Genome Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Open access
- Published: 21 June 2021
The lived experience of people with obesity: study protocol for a systematic review and synthesis of qualitative studies
- Emma Farrell ORCID: orcid.org/0000-0002-7780-9428 1 ,
- Marta Bustillo 2 ,
- Carel W. le Roux 3 ,
- Joe Nadglowski 4 ,
- Eva Hollmann 1 &
- Deirdre McGillicuddy 1
Systematic Reviews volume 10 , Article number: 181 ( 2021 ) Cite this article
6480 Accesses
9 Altmetric
Metrics details
Obesity is a prevalent, complex, progressive and relapsing chronic disease characterised by abnormal or excessive body fat that impairs health and quality of life. It affects more than 650 million adults worldwide and is associated with a range of health complications. Qualitative research plays a key role in understanding patient experiences and the factors that facilitate or hinder the effectiveness of health interventions. This review aims to systematically locate, assess and synthesise qualitative studies in order to develop a more comprehensive understanding of the lived experience of people with obesity.
This is a protocol for a qualitative evidence synthesis of the lived experience of people with obesity. A defined search strategy will be employed in conducting a comprehensive literature search of the following databases: PubMed, Embase, PsycInfo, PsycArticles and Dimensions (from 2011 onwards). Qualitative studies focusing on the lived experience of adults with obesity (BMI >30) will be included. Two reviewers will independently screen all citations, abstracts and full-text articles and abstract data. The quality of included studies will be appraised using the critical appraisal skills programme (CASP) criteria. Thematic synthesis will be conducted on all of the included studies. Confidence in the review findings will be assessed using GRADE CERQual.
The findings from this synthesis will be used to inform the EU Innovative Medicines Initiative (IMI)-funded SOPHIA (Stratification of Obesity Phenotypes to Optimize Future Obesity Therapy) study. The objective of SOPHIA is to optimise future obesity treatment and stimulate a new narrative, understanding and vocabulary around obesity as a set of complex and chronic diseases. The findings will also be useful to health care providers and policy makers who seek to understand the experience of those with obesity.
Systematic review registration
PROSPERO CRD42020214560 .
Peer Review reports
Obesity is a complex chronic disease in which abnormal or excess body fat (adiposity) impairs health and quality of life, increases the risk of long-term medical complications and reduces lifespan [ 1 ]. Operationally defined in epidemiological and population studies as a body mass index (BMI) greater than or equal to 30, obesity affects more than 650 million adults worldwide [ 2 ]. Its prevalence has almost tripled between 1975 and 2016, and, globally, there are now more people with obesity than people classified as underweight [ 2 ].
Obesity is caused by the complex interplay of multiple genetic, metabolic, behavioural and environmental factors, with the latter thought to be the proximate factor which enabled the substantial rise in the prevalence of obesity in recent decades [ 3 , 4 ]. This increased prevalence has resulted in obesity becoming a major public health issue with a resulting growth in health care and economic costs [ 5 , 6 ]. At a population level, health complications from excess body fat increase as BMI increases [ 7 ]. At the individual level, health complications occur due to a variety of factors such as distribution of adiposity, environment, genetic, biologic and socioeconomic factors [ 8 ]. These health complications include type 2 diabetes [ 9 ], gallbladder disease [ 10 ] and non-alcoholic fatty liver disease [ 11 ]. Excess body fat can also place an individual at increased cardiometabolic and cancer risk [ 12 , 13 , 14 ] with an estimated 20% of all cancers attributed to obesity [ 15 ].
Although first recognised as a disease by the American Medical Association in 2013 [ 16 ], the dominant cultural narrative continues to present obesity as a failure of willpower. People with obesity are positioned as personally responsible for their weight. This, combined with the moralisation of health behaviours and the widespread association between thinness, self-control and success, has resulted in those who fail to live up to this cultural ideal being subject to weight bias, stigma and discrimination [ 17 , 18 , 19 ]. Weight bias, stigma and discrimination have been found to contribute, independent of weight or BMI, to increased morbidity or mortality [ 20 ].
Thomas et al. [ 21 ] highlighted, more than a decade ago, the need to rethink how we approach obesity so as not to perpetuate damaging stereotypes at a societal level. Obesity research then, as now, largely focused on measurable outcomes and quantifiable terms such as body mass index [ 22 , 23 ]. Qualitative research approaches play a key role in understanding patient experiences, how factors facilitate or hinder the effectiveness of interventions and how the processes of interventions are perceived and implemented by users [ 24 ]. Studies adopting qualitative approaches have been shown to deliver a greater depth of understanding of complex and socially mediated diseases such as obesity [ 25 ]. In spite of an increasing recognition of the integral role of patient experience in health research [ 25 , 26 ], the voices of patients remain largely underrepresented in obesity research [ 27 , 28 ].
Systematic reviews and syntheses of qualitative studies are recognised as a useful contribution to evidence and policy development [ 29 ]. To the best of the authors’ knowledge, this will be the first systematic review and synthesis of qualitative studies focusing on the lived experience of people with obesity. While systematic reviews have been carried out on patient experiences of treatments such as behavioural management [ 30 ] and bariatric surgery [ 31 ], this review and synthesis will be the first to focus on the experience of living with obesity rather than patient experiences of particular treatments or interventions. This focus represents a growing awareness that ‘patients have a specific expertise and knowledge derived from lived experience’ and that understanding lived experience can help ‘make healthcare both effective and more efficient’ [ 32 ].
This paper outlines a protocol for the systematic review of qualitative studies based on the lived experience of people with obesity. The findings of this review will be synthesised in order to develop an overview of the lived experience of patients with obesity. It will look, in particular, at patient concerns around the risks of obesity and their aspirations for response to obesity treatment.
The review protocol has been registered within the PROSPERO database (registration number: CRD42020214560) and is being reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) statement [ 33 , 34 ] (see checklist in Additional file 1 ).
Information sources and search strategy
The primary source of literature will be a structured search of the following electronic databases (from January 2011 onwards—to encompass the increase in research focused on patient experience observed over the last 10 years): PubMed, Embase, PsycInfo, PsycArticles and Dimensions. There is no methodological agreement as to how many search terms or databases out to be searched as part of a ‘good’ qualitative synthesis (Toye et al. [ 35 ]). However, the breadth and depth of the search terms, the inclusion of clinical and personal language and the variety within the selected databases, which cover areas such as medicine, nursing, psychology and sociology, will position this qualitative synthesis as comprehensive. Grey literature will not be included in this study as its purpose is to conduct a comprehensive review of peer-reviewed primary research. The study’s patient advisory board will be consulted at each stage of the review process, and content experts and authors who are prolific in the field will be contacted. The literature searches will be designed and conducted by the review team which includes an experienced university librarian (MB) following the methodological guidance of chapter two of the JBI Manual for Evidence Synthesis [ 36 ]. The search will include a broad range of terms and keywords related to obesity and qualitative research. A full draft search strategy for PubMed is provided in Additional file 2 .
Eligibility criteria
Studies based on primary data generated with adults with obesity (operationally defined as BMI >30) and focusing on their lived experience will be eligible for inclusion in this synthesis (Table 1 ). The context can include any country and all three levels of care provision (primary, secondary and tertiary). Only peer-reviewed, English language, articles will be included. Studies adopting a qualitative design, such as phenomenology, grounded theory or ethnography, and employing qualitative methods of data collection and analysis, such as interviews, focus groups, life histories and thematic analysis, will be included. Publications with a specific focus, for example, patient’s experience of bariatric surgery, will be included, as well as studies adopting a more general view of the experience of obesity.
Screening and study selection process
Search results will be imported to Endnote X9, and duplicate entries will be removed. Covidence [ 38 ] will be used to screen references with two reviewers (EF and EH) removing entries that are clearly unrelated to the research question. Titles and abstracts will then be independently screened by two reviewers (EF and EH) according to the inclusion criteria (Table 1 ). Any disagreements will be resolved through a third reviewer (DMcG). This layer of screening will determine which publications will be eligible for independent full-text review by two reviewers (EF and EH) with disagreements again being resolved by a third reviewer (DMcG).
Data extraction
Data will be extracted independently by two researchers (EF and EH) and combined in table format using the following headings: author, year, title, country, research aims, participant characteristics, method of data collection, method of data analysis, author conclusions and qualitative themes. In the case of insufficient or unclear information in a potentially eligible article, the authors will be contacted by email to obtain or confirm data, and a timeframe of 3 weeks to reply will be offered before article exclusion.
Quality appraisal of included studies
This qualitative synthesis will facilitate the development of a conceptual understanding of obesity and will be used to inform the development of policy and practice. As such, it is important that the studies included are themselves of suitable quality. The methodological quality of all included studies will be assessed using the critical appraisal skills programme (CASP) checklist, and studies that are deemed of insufficient quality will be excluded. The CASP checklist for qualitative research comprises ten questions that cover three main issues: Are the results of the study under review valid? What are the results? Will the results help locally? Two reviewers (EF and EH) will independently evaluate each study using the checklist with a third and fourth reviewer (DMcG and MB) available for consultation in the event of disagreement.
Data synthesis
The data generated through the systematic review outlined above will be synthesised using thematic synthesis as described by Thomas and Harden [ 39 ]. Thematic synthesis enables researchers to stay ‘close’ to the data of primary studies, synthesise them in a transparent way and produce new concepts and hypotheses. This inductive approach is useful for drawing inference based on common themes from studies with different designs and perspectives. Thematic synthesis is made up of a three-step process. Step one consists of line by line coding of the findings of primary studies. The second step involves organising these ‘free codes’ into related areas to construct ‘descriptive’ themes. In step three, the descriptive themes that emerged will be iteratively examined and compared to ‘go beyond’ the descriptive themes and the content of the initial studies. This step will generate analytical themes that will provide new insights related to the topic under review.
Data will be coded using NVivo 12. In order to increase the confirmability of the analysis, studies will be reviewed independently by two reviewers (EF and EH) following the three-step process outlined above. This process will be overseen by a third reviewer (DMcG). In order to increase the credibility of the findings, an overview of the results will be brought to a panel of patient representatives for discussion. Direct quotations from participants in the primary studies will be italicised and indented to distinguish them from author interpretations.
Assessment of confidence in the review findings
Confidence in the evidence generated as a result of this qualitative synthesis will be assessed using the Grading of Recommendations Assessment, Development and Evaluation Confidence in Evidence from Reviews of Qualitative Research (GRADE CERQual) [ 40 ] approach. Four components contribute to the assessment of confidence in the evidence: methodological limitations, relevance, coherence and adequacy of data. The methodological limitations of included studies will be examined using the CASP tool. Relevance assesses the degree to which the evidence from the primary studies applies to the synthesis question while coherence assesses how well the findings are supported by the primary studies. Adequacy of data assesses how much data supports a finding and how rich this data is. Confidence in the evidence will be independently assessed by two reviewers (EF and EH), graded as high, moderate or low, and discussed collectively amongst the research team.
Reflexivity
For the purposes of transparency and reflexivity, it will be important to consider the findings of the qualitative synthesis and how these are reached, in the context of researchers’ worldviews and experiences (Larkin et al, 2019). Authors have backgrounds in health science (EF and EH), education (DMcG and EF), nursing (EH), sociology (DMcG), philosophy (EF) and information science (MB). Prior to conducting the qualitative synthesis, the authors will examine and discuss their preconceptions and beliefs surrounding the subject under study and consider the relevance of these preconceptions during each stage of analysis.
Dissemination of findings
Findings from the qualitative synthesis will be disseminated through publications in peer-reviewed journals, a comprehensive and in-depth project report and presentation at peer-reviewed academic conferences (such as EASO) within the field of obesity research. It is also envisaged that the qualitative synthesis will contribute to the shared value analysis to be undertaken with key stakeholders (including patients, clinicians, payers, policy makers, regulators and industry) within the broader study which seeks to create a new narrative around obesity diagnosis and treatment by foregrounding patient experiences and voice(s). This synthesis will be disseminated to the 29 project partners through oral presentations at management board meetings and at the general assembly. It will also be presented as an educational resource for clinicians to contribute to an improved understanding of patient experience of living with obesity.
Obesity is a complex chronic disease which increases the risk of long-term medical complications and a reduced quality of life. It affects a significant proportion of the world’s population and is a major public health concern. Obesity is the result of a complex interplay of multiple factors including genetic, metabolic, behavioural and environmental factors. In spite of this complexity, obesity is often construed in simple terms as a failure of willpower. People with obesity are subject to weight bias, stigma and discrimination which in themselves result in increased risk of mobility or mortality. Research in the area of obesity has tended towards measurable outcomes and quantitative variables that fail to capture the complexity associated with the experience of obesity. A need to rethink how we approach obesity has been identified—one that represents the voices and experiences of people living with obesity. This paper outlines a protocol for the systematic review of available literature on the lived experience of people with obesity and the synthesis of these findings in order to develop an understanding of patient experiences, their concerns regarding the risks associated with obesity and their aspirations for response to obesity treatment. Its main strengths will be the breadth of its search remit—focusing on the experiences of people with obesity rather than their experience of a particular treatment or intervention. It will also involve people living with obesity and its findings disseminated amongst the 29 international partners SOPHIA research consortium, in peer reviewed journals and at academic conferences. Just as the study’s broad remit is its strength, it is also a potential challenge as it is anticipated that searchers will generate many thousands of results owing to the breadth of the search terms. However, to the best of the authors’ knowledge, this will be the first systematic review and synthesis of its kind, and its findings will contribute to shaping the optimisation of future obesity understanding and treatment.
Availability of data and materials
Not applicable.
Abbreviations
Body mass index
Critical appraisal skills programme
Grading of Recommendations Assessment, Development and Evaluation Confidence in Evidence from Reviews of Qualitative Research
Innovative Medicines Initiative
Medical Subject Headings
Population, phenomenon of interest, context, study type
Stratification of Obesity Phenotypes to Optimize Future Obesity Therapy
Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 2020;192(31):E875–91. https://doi.org/10.1503/cmaj.191707 .
Article Google Scholar
World Health Organisation. Fact sheet: obesity and overweight. Geneva: World Health Organisation; 2020.
Google Scholar
Mechanick J, Hurley D, Garvey W. Adiposity-based chronic disease as a new diagnostic term: the American Association of Clinical Endocrinologists and American College Of Endocrinology position statement. Endocrine Pract. 2017;23(3):372–8. https://doi.org/10.4158/EP161688.PS .
Garvey W, Mechanick J. Proposal for a scientifically correct and medically actionable disease classification system (ICD) for obesity. Obesity. 2020;28(3):484–92. https://doi.org/10.1002/oby.22727 .
Article PubMed Google Scholar
Biener A, Cawley J, Meyerhoefer C. The high and rising costs of obesity to the US health care system. J Gen Intern Med. 2017;32(Suppl 1):6–8. https://doi.org/10.1007/s11606-016-3968-8 .
Article PubMed PubMed Central Google Scholar
Department of Health and Social Care. Healthy lives, healthy people: a call to action on obesity in England. London: Department of Health and Social Care; 2011.
Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. https://doi.org/10.1016/S0140-6736(16)30175-1 .
Sharma AM. M, M, M & M: a mnemonic for assessing obesity. Obesity Reviews. 2010;11(11):808–9. https://doi.org/10.1111/j.1467-789X.2010.00766.x .
Article PubMed CAS Google Scholar
Asnawi A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89:309-19. Diab Res Clin Pract. 2010;89:309–19.
Dagfinn A, Teresa N, Lars JV. Body mass index, abdominal fatness and the risk of gallbladder disease. 2015;30(9):1009.
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9).
Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. 2003;289(2):187-193.
Grover SA, Kaouache M, Rempel P, Joseph L, Dawes M, Lau DCW, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. 2015;3(2):114-122.
Ackerman S, Blackburn O, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6(2):195–203. https://doi.org/10.1007/s13679-017-0263-x .
Wolin K, Carson K, Colditz G. Obesity and cancer. Oncol. 2010;15(6):556–65. https://doi.org/10.1634/theoncologist.2009-0285 .
Resolution 420: Recognition of obesity as a disease [press release]. 05/16/13 2013.
Brownell KD. Personal responsibility and control over our bodies: when expectation exceeds reality. 1991;10(5):303-10.
Puhl RM, Latner JD, O'Brien K, Luedicke J, Danielsdottir S, Forhan M. A multinational examination of weight bias: predictors of anti-fat attitudes across four countries. 2015;39(7):1166-1173.
Browne NT. Weight bias, stigmatization, and bullying of obese youth. 2012;7(3):107-15.
Sutin AR, Stephan Y, Terracciano A. Weight discrimination and risk of mortality. 2015;26(11):1803-11.
Thomas SL, Hyde J, Karunaratne A, Herbert D, Komesaroff PA. Being “fat” in today’s world: a qualitative study of the lived experiences of people with obesity in Australia. 2008;11(4):321-30.
Ogden K, Barr J, Rossetto G, Mercer J. A “messy ball of wool”: a qualitative study of the dimensions of the lived experience of obesity. 2020;8(1):1-14.
Ueland V, Furnes B, Dysvik E, R¯rtveit K. Living with obesity-existential experiences. 2019;14(1):1-12.
Avenell A, Robertson C, Skea Z, Jacobsen E, Boyers D, Cooper D, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. 2018;22(68).
The PLoS Medicine Editors. Qualitative research: understanding patients’ needs and experiences. Plos Med. 2007;4(8):1283–4.
Boulton M, Fitzpatrick R. Qualitative methods for assessing health care doi:10.1136/qshc.3.2.107. Qual Health Care. 1994;3:107–13.
Johnstone J, Herredsberg C, Lacy L, Bayles P, Dierking L, Houck A, et al. What I wish my doctor really knew: the voices of patients with obesity. Ann Fam Med. 2020;18(2):169–71. https://doi.org/10.1370/afm.2494 .
Brown I, Thompson J, Tod A, Jones G. Primary care support for tackling obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract. 2006;56(530):666–72.
PubMed PubMed Central Google Scholar
Brown I, Gould J. Qualitative studies of obesity: a review of methodology. Health. 2013;5(8A3):69–80.
Garip G, Yardley L. A synthesis of qualitative research on overweight and obese people’s views and experiences of weight management. Clin Obes. 2011;1(2-3):10–126.
Coulman K, MacKichan F, Blazeby J, Owen-Smith A. Patient experiences of outcomes of bariatric surgery: a systematic review and qualitative synthesis. Obes Rev. 2017;18(5):547–59. https://doi.org/10.1111/obr.12518 .
European Patients’ Forum. “Patients’ Perceptions of Quality in Healthcare”: Report of a survey conducted by EPF in 2016 Brussels: European Patients’ Forum; 2017.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(jan02 1):g7647. https://doi.org/10.1136/bmj.g7647 .
Toye F, et al. Meta-ethnography 25 years on: challenges and insights for synthesising a large number of qualitative studies. BMC Med Res Methodol. 2014;14(80).
Lockwood C, Porrit K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, et al. Chapter 2: Systematic reviews of qualitative evidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis: JBI; 2020, doi: https://doi.org/10.46658/JBIMES-20-03 .
Methley AM, et al. PICO, PICOS and SPIDER: a comparison study of spcificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Services Res. 2014;14.
Covidence. Cochrane Community; 2020. Available from: https://www.covidence.org .
Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8(1):45. https://doi.org/10.1186/1471-2288-8-45 .
Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13(1):2. https://doi.org/10.1186/s13012-017-0688-3 .
Download references
Acknowledgements
Any amendments made to this protocol when conducting the study will be outlined in PROSPERO and reported in the final manuscript.
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 875534. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and T1D Exchange, JDRF and Obesity Action Coalition. The funding body had no role in the design of the study and will not have a role in collection, analysis and interpretation of data or in writing the manuscript.
Author information
Authors and affiliations.
School of Education, University College Dublin, Belfield, Dublin 4, Ireland
Emma Farrell, Eva Hollmann & Deirdre McGillicuddy
University College Dublin Library, Dublin, Ireland
Marta Bustillo
Diabetes Complications Research Centre, University College Dublin, Dublin, Ireland
Carel W. le Roux
Obesity Action Coalition, Tampa, USA
Joe Nadglowski
You can also search for this author in PubMed Google Scholar
Contributions
EF conceptualised and designed the protocol with input from DMcG and MB. EF drafted the initial manuscript. EF and MB defined the concepts and search items with input from DmcG, CleR and JN. MB and EF designed and executed the search strategy. DMcG, CleR, JN and EH provided critical insights and reviewed and revised the protocol. All authors have approved and contributed to the final written manuscript.
Corresponding author
Correspondence to Emma Farrell .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:..
PRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol*.
Additional file 2: Table 1
. Search PubMed search string.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Farrell, E., Bustillo, M., le Roux, C.W. et al. The lived experience of people with obesity: study protocol for a systematic review and synthesis of qualitative studies. Syst Rev 10 , 181 (2021). https://doi.org/10.1186/s13643-021-01706-5
Download citation
Received : 28 October 2020
Accepted : 14 May 2021
Published : 21 June 2021
DOI : https://doi.org/10.1186/s13643-021-01706-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lived experience
- Patient experience
- Obesity treatment
- Qualitative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Obesity Synthesis Essay. As an apathetic killer of 300,000 innocent individuals in America, obesity is one of the largest controversial diseases of discussion in America's history. Although obesity itself isn't the direct cause of these numerous deaths, it is the lead contributor in augmenting the chance of the incidence of heart disease ...
Synthesis Essay On Obesity; Synthesis Essay On Obesity. 899 Words 4 Pages. The persistent crisis of food and fitness and its effects on people's well-being has been a long-term concern that has risen more prominently in the last couple of years. While controlling eating habits and physical activity is mainly under the control of the person it ...
Synthesis Essay Obesity. Society should be involved up to a certain point, and should have more realistic figures to inspire the youth. Barbie, an unrealistically proportioned doll, should not be the inspiration for the youth, yet it has been a cultural icon since 1959 (Source E). Since 1959, society has been focusing on the woman's figure and ...
Ap English synthesis essay obesity. In the United States the society needs to work on controlling this problem known as obesity. It is a problem that if does not get controlled sooner than later, will spiral out of hand even more. Before this spirals out of control society needs to take action to reducing the cost of healthy food amongst middle ...
Introduction. Obesity is defined as having a body mass index (BMI) of 30 or higher. In America, the prevalence of obesity has been steadily increasing over the past few decades, with currently around 42% of the population being classified as obese. Addressing this issue is significant as it has far-reaching impacts on both individual and ...
This essay will explore the various causes of obesity and their effects on individuals and society as a whole. One of the primary causes of obesity is dietary habits and nutritional intake. The consumption of high-calorie, low-nutrient foods, such as fast food, sugary beverages, and processed snacks, has become increasingly prevalent in modern ...
Obesity has become a global epidemic and is one of today's most public health problems worldwide. Obesity poses a major risk for a variety of serious diseases including diabetes mellitus, non-alcoholic liver disease (NAFLD), cardiovascular disease, hypertension and stroke, and certain forms of cancer (Bluher, 2019).Obesity is mainly caused by imbalanced energy intake and expenditure due to a ...
AP Language Synthesis Essay: Childhood Obesity Source F: McElroy Apart from the profit (or funding) motive, political bias may be playing a role at the CDC and with other obesity research. In January 1998, the editors of the New England Journal of Medicine cast a skeptical eye on the "300,000 deaths" from obesity per year figure and
Obesity Synthesis Exercise Directions: The following assignment is based on seven sources. This question requires you to ... In a well-written essay synthesizing at least four of the seven sources, develop a position on the extent of the role society and/or government should play in the obesity issue.
Structuring your synthesis essay by topic works best for more complicated ideas with different aspects that should be explored individually. Example outline: I. Introduction A. Thesis statement. II. Topic 1 A. Source A discussing Topic 1 1. A point or piece of evidence/data from Source A about Topic 1 2.
Essay on obesity! Find high quality essays on 'Obesity' especially written for school, college, science and medical students. These essays will also guide you to learn about the causes, factors, treatment, management and complications related to obesity. Obesity is a chronic health condition in which the body fat reaches abnormal level.
Synthesis Essay On Obesity. Improved Essays. 527 Words; 3 Pages; Open Document. Essay Sample Check Writing Quality. Show More. Obesity is determined by dividing an individual's weight and height together to calculate their body mass index (BMI). A person's BMI is a measure of the amount of body fat one person has, and it determines whether ...
Successful evidence gathering, evaluation, and synthesis for use in obesity prevention usually require the involvement of a number of disciplines using a variety of methodologies and technical languages. The framework incorporates a standardized approach using a uniform language and structure for summarizing the relevant evidence in a ...
636 Words. 3 Pages. Open Document. Obesity is a medical condition in which excess fats have grown and accumulated to the point of harm, and a shortened life expectancy. Obesity is a problem that has been more prominent in the 20th and 21st century and is now a main concern for both society and the government. It is the citizen's duty to ...
Some genetic and lifestyle factors affect an individual's likelihood of adult obesity; thus, the significant clusters of obesity observed in specific geographical regions and contexts also signal the impact of socioeconomic and environmental factors in "obesogenic" environments [13].Understanding the causes and determinants of obesity is a critical step toward creating effective policy and ...
Synthesis Essay Obesity. 680 Words 3 Pages. Kal Yewlsew Boswell Hon English 10 Feb 21, 2024. In order to decrease childhood obesity and the way they're influenced, the way advertisements are presented needs to be changed too. Children will give all their attention to something that interests them and the way advertisements are marketed ...
1 INTRODUCTION. Obesity is a complex chronic disease in which abnormal or excess body fat (adiposity) impairs health and quality of life, increases the risk of long-term medical complications, and reduces lifespan. 1, 2 An estimated 650 million people are affected worldwide, and the prevalence of obesity has almost tripled since 1975. 3 Obesity is caused by the complex interplay of multiple ...
This updated synthesis of obesity prevention interventions for children aged 6-18 years, found a small beneficial impact on child BMI for school-based obesity prevention interventions. A more comprehensive assessment of interventions is required to identify mechanisms of effective interventions to inform future obesity prevention public health policy, which may be particularly salient in for ...
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people's health. ... ADCY3 encodes an adenylate cyclase that catalyses the synthesis of cAMP, an ...
Obesity is a prevalent, complex, progressive and relapsing chronic disease characterised by abnormal or excessive body fat that impairs health and quality of life. It affects more than 650 million adults worldwide and is associated with a range of health complications. Qualitative research plays a key role in understanding patient experiences and the factors that facilitate or hinder the ...
Synthesis Essay Obesity. 632 Words; 3 Pages; Synthesis Essay Obesity. In recent discussion of obesity, a controversial issue has been whether there should be involvement of society and the government. On one hand, some argue that obese people harm everyone, therefore, everyone should be involved. One the other hand, people argue that obese ...