A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- General Committee-Related Information
- ACIP Work Groups
- ACIP Meeting Information
- ACIP Recommendations
- Apply for ACIP Membership
- ACIP Committee Members
- Evidence-Based Recommendations—GRADE
- GRADE Evidence Tables – Recommendations in MMWR
- Evidence to Recommendations Frameworks
Related Topics:
- View All Home
- ACIP GRADE Handbook
- Vaccine-Specific Recommendations
- Vaccines & Immunizations

ACIP Evidence to Recommendations for Use of Moderna COVID-19 Vaccine
The Evidence to Recommendations (EtR) frameworks describe information considered in moving from evidence to ACIP vaccine recommendations.
Question: Should vaccination with the Moderna COVID-19 vaccine (Spikevax, 2-dose primary series) be recommended for persons 18 years of age and older?
Population: Persons 18 years of age and older
- Symptomatic laboratory-confirmed COVID-19
- Hospitalization due to COVID-19
- Death due to COVID-19
- Asymptomatic SARS-CoV-2 infection
- Serious Adverse Events (SAEs) (including myocarditis and anaphylaxis)
- Reactogenicity (proportion with grade 3 or worse reactions)
The emergence of SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), in late 2019 has led to a global pandemic with dramatic societal and economic impact on individual persons and communities. In the United States, more than 76 million cases and more than 900,000 COVID-19-associated deaths have been reported as of February 7, 2022. Persons of all ages are at risk for infection and severe disease. However, the risk for severe illness from COVID-19 is higher in people aged ≥65 years, those living in long-term care facilities, and those with chronic medical conditions. Additionally, there is a disproportionate burden of COVID-19 infections and deaths among racial and ethnic minority communities. Non-Hispanic Black, Hispanic/Latino (Hispanic) and American Indian/Alaska Native persons have experienced higher rates of disease, hospitalization and death compared with non-Hispanic White persons. This is likely related to inequities in social determinants of health that put racial and ethnic minority groups at increased risk for COVID-19, including overrepresentation among essential workers who have higher risk of exposure to COVID-19, lower incomes, reduced access to healthcare, or higher rates of comorbid conditions.
In the United States, the first vaccines to prevent COVID-19 received Food and Drug Administration (FDA) Emergency Use Authorizations (EUA): Pfizer-BioNTech on December 11, 2020, for persons aged 16 years and older, Moderna on December 18, 2020, for adults aged 18 years and older, and Janssen on February 27, 2021, for adults aged 18 years and older. On August 23, 2021, the FDA approved a Biologics License Application (BLA) for use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 16 years and older; and on January 31, 2022, the FDA approved a BLA for use of the Moderna COVID-19 vaccine in persons aged 18 years and older.
Additional background information supporting the ACIP recommendation on the use of Moderna COVID-19 vaccine can be found in the relevant publication of the recommendation referenced on the ACIP website .
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| Is the problem of public health importance? | Yes | COVID-19 is a major global public health threat that dramatically disrupted all sectors of society worldwide. In the United States, COVID-19 has important associated morbidity and mortality. As of February 7, 2022, there were 76,782,002 COVID-19 cases reported in the United States for an incidence of 23,425 cases per 100,000 population. Among sites participating in population-based surveillance for laboratory-confirmed COVID-19-associated hospitalizations, the overall cumulative hospitalization rate between March 1, 2020 and January 29, 2022 was 898 per 100,000 population. Among those hospitalized, 23.5% required care in an intensive care unit and 13.5% died. As of February 7, 2022, there were 903,038 COVID-19-associated deaths reported in the United States. | As of February 3, 2022, 29 states had over 80% intensive care unit (ICU) beds occupied. As of February 8, 2022, more than 540 million doses of COVID-19 vaccines had been administered in the United States. However, 25.6% of people ≥18 years of age were not fully vaccinated. Vaccination coverage varies by geography and age. Older adults ≥18 had a higher proportion of individuals receiving ≥1 dose (95% in 65-74 years) compared to younger persons 12-17 (66.7%). As of February 5, 2022, the Omicron variant is the dominant circulating variant in the United States and spreads more easily than the original virus that causes COVID-19 and the Delta variant. |
Benefits and Harms
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| How substantial are the desirable anticipated effects? | Large | In the Phase III randomized controlled trial (RCT), the Moderna COVID-19 vaccine demonstrated efficacy up to 5 months after vaccination. The overall efficacy* against symptomatic laboratory-confirmed COVID-19 was 92.7 (95% confidence interval [CI] 90.4–94.4%)) ( ). For hospitalization due to COVID-19, 25 events occurred, 24 in the placebo group and 1 in the vaccine group. Vaccine efficacy against hospitalization due to COVID-19 was 95.9% (95% CI 69.5–99.4%) ( ). Deaths due to COVID-19 were uncommon, zero in the vaccine group and three in the placebo group (vaccine efficacy: 100%) ( ). | Thirty-three publications, which reported data on 31 studies or surveillance systems, were included in the evidence synthesis and GRADE evidence assessment. Data were reviewed from five RCTs publications including two publications from the Phase I trial, one publication from a Phase II trial, and two publications from the Phase III trial. Data were reviewed from 26 vaccine effectiveness studies. |
| How substantial are the undesirable anticipated effects? | Small | In the Phase III RCT, numbers of serious adverse events (SAEs) were comparable between the vaccine group and the placebo group across the two RCTs (Phase III: 268/15,184 (1.8%) vs. 292/15,164 (1.9%); Phase II: 0/200 (0.0%) vs. 0/200 (0.0%)); there were no cases of vaccine-associated enhanced disease or vaccine-related deaths ( ). Grade ≥3 reactions** generally were not uncommon and occurred more frequently in the vaccine than placebo groups ( ). Observational data on serious adverse events were reviewed. A rapid cycle analysis from Vaccine Safety Datalink (VSD) evaluated chart-reviewed cases of myocarditis occurring among persons aged 18–39 years in a 7-day risk interval following dose 2 of the Moderna COVID-19 vaccine versus a 22–42 day comparison interval resulting in an adjusted rate ratio of 18.8 (95% CI 6.7–64.9) ( ). Data from Vaccine Adverse Event Reporting System showed an elevated ratio of observed to expected myocarditis cases in the 7-day interval following vaccination among females aged 18–29 years and among males aged 18–49 years, with higher observed/expected ratios in males than females. A rapid cycle analysis of data from VSD evaluated chart-reviewed cases of anaphylaxis among all vaccinated persons aged ≥18 years. Based on events occurring in a 0–1 day risk interval after vaccination, the estimated incidence of confirmed anaphylaxis was 5.1 (95% CI 3.3–7.6) per million doses. | Safety data showed an acceptable safety profile. In post-authorization safety monitoring, myocarditis and anaphylaxis were rare but more common following vaccination. |
| Do the desirable effects outweigh the undesirable effects? | Favors intervention | The Work Group felt that the desirable effects of the Moderna COVID-19 vaccine outweigh the undesirable effects. | |
| What is the overall certainty of this evidence for the critical outcomes? | High to moderate | For the critical outcomes, the certainty of evidence was high for prevention of symptomatic laboratory-confirmed COVID-19, moderate for prevention of hospitalizations due to COVID-19, and moderate for serious adverse events. For important outcomes, the certainty of evidence was moderate for prevention of death due to COVID-19, high for prevention of asymptomatic infection, and high for reactogenicity. |
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| Does the target population feel that the desirable effects are large relative to undesirable effects? | Large | In 63 national surveys among U.S. adults conducted between December 2020 and January 2022, for the scenario that a vaccine would be or had been approved in the United States, acceptability was moderate overall. The proportion intending to receive the COVID-19 vaccine ranged across the surveys between 47%-84%. Vaccination intent over time has been relatively stable. A survey conducted from January 2 – 8, 2022 reported 84.7% of adults ages 18 years and older are vaccinated or definitely will get vaccinated. Furthermore, when unvaccinated adults were asked what, if anything, would convince them to get vaccinated for COVID-19, about half (48%) said nothing would convince them to get a COVID-19 vaccine. | Knowledge and attitudes may change with time, and intentions may not reflect uptake. The survey sample populations may not be representative, limiting the generalizability of the results to all adults in the U.S. Most surveys used convenience sampling, had limited representation of minority and priority populations (such as healthcare workers or essential workers), and low or unknown response rates. |
| Is there important uncertainty about or variability in how much people value the main outcomes? | Probably important uncertainty or variability | In a recent ongoing survey to assess vaccination intention of unvaccinated Americans in response to the FDA’s Biologics License Application for the Moderna COVID-19 vaccine, only 5% of unvaccinated respondents said they would get a COVID-19 vaccine as soon as they could if the Moderna vaccine received full approval from the FDA. Moreover, 20% said they would continue waiting to see if COVID vaccines were safe and effective, and 52% said they would definitely not get vaccinated or would only do so if it were required. | During the data collection period (January 27 – January 31, 2022), 29% of unvaccinated respondents thought the Moderna COVID-19 vaccine had already received full approval from the FDA. |
Acceptability
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| Is the intervention acceptable to key stakehold-ers? | Yes | Pandemic vaccination response planning requires collaboration among a wide range of public- and private-sector partners. COVID-19 vaccination has been implemented in a variety of settings, including state and local health departments, healthcare sites and hospitals, mass vaccination clinics, Long Term Care Facilities, and retail pharmacies. As of February 9, 2022, more than 205 million doses have been administered. | Vaccination with the Moderna COVID-19 vaccine was already highly acceptable to stakeholders under FDA EUA and ACIP interim recommendation, and vaccination may be more acceptable to stakeholders under full FDA approval and a standard ACIP recommendation. |
Feasibility
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| Is the intervention feasible to implement? | Yes | There are a variety of barriers that are likely to limit the feasibility of implementing the Moderna COVID-19 vaccine including: C to -15 C (-58 to 5 F). The vaccine should not be stored on dry ice or below -50 C (-58 F) and the vaccine must be stored in the original carton to protect the vials from light. Vials can be refrigerated between 2 C to 8 C (36 to 46 F) for up to 30 days prior to first use. After the first dose has been withdrawn, the vial should be held between 2 C to 25 C (36 to 77 F), and vials should be discarded 12 hours after the first puncture. | The Work Group determined that the Moderna COVID-19 vaccine is feasible to implement. |
Resource Use
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| Is the intervention a reasonable and efficient allocation of resources? | Yes | A recent study estimated that preventable COVID-19 hospitalizations among unvaccinated adults in the United States cost over $13 billion from June to November in 2021. An effective vaccine combined with a successful vaccination program would be expected to reduce costs associated with COVID-19 disease outcomes and other COVID-19 mitigation activities. Several published modeling studies have found that COVID-19 vaccinations are likely to be of a reasonable economic value and may also be cost-saving under many circumstances. |
| Criteria | Work Group Judgements | Evidence | Additional Information |
|---|---|---|---|
| What would be the impact of the intervention on health equity? | Probably no impact | As of January 22, 2022, cumulative COVID-19-associated hospitalizations in the United States illustrated that rates (per 100,000 population) were higher among American Indian/Alaska Native, Black, and Hispanic populations compared to White and Asian/Pacific Islanders. Further analysis highlights disparities in vaccine intent by geographic location. Vaccine uptake lags in adults living in rural and suburban areas compared with urban areas. As of November 21, 2021, eight in ten urban residents (79%) say they have received at least one dose of a COVID-19 vaccine, compared to seven in ten suburban adults and 67% of rural adults. Moreover, one in five (21%) of those living in rural areas and one in six (16%) of those living in suburban areas say they will “definitely not” get a COVID-19 vaccine, at least twice the share of urban residents who say the same (8%). | The Work Group determined a standard ACIP recommendation for Moderna COVID-19 vaccine would probably have no impact on equity. |
Balance of consequences
Desirable consequences clearly outweigh undesirable consequences in most settings.
Is there sufficient information to move forward with a recommendation? Yes.
Policy options for ACIP consideration
ACIP recommends the intervention
Draft recommendation (text)
The Moderna COVID-19 vaccine is recommended for people 18 years of age and older under FDA’s Biologics License Application.
Final deliberation and decision by the ACIP
Final acip recommendation.
ACIP recommends the intervention.
The Moderna COVID-19 vaccine is recommended for persons 18 years of age and older in the U.S. population under the FDA’s Biologics License Application.
*Overall vaccine efficacy was calculated at ≥14 days after second dose of vaccine among persons without evidence of prior SARS-CoV-2 infection.
†Asymptomatic SARS-CoV-2 infection is defined as (1) positive serology (non-spike protein), and (2) no prior SARS-CoV-2 positive PCR or COVID-19 symptoms during the study. Seroconversion to a non-spike protein can distinguish between natural infection and vaccine-induced immunity.
**Grade 3 reactions are defined as: pain at injection site or axillary swelling/tenderness that prevents daily activity, redness > 10 cm, and swelling > 10 cm; fever 102.1-104.0°F (39°C–40°C); vomiting that requires intravenous hydration; or headache, fatigue/tiredness, new or worsened muscle pain, or new or worsened joint pain that prevents daily routine activity; grade 4 reactions are defined as: requires emergency room visit or hospitalization, fever >104°F (40°C).
§§Serious adverse events defined as any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, or results in persistent disability/incapacity.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#trends_totalcases_totalcasesper100k . Accessed: February 9, 2022.
- COVID-NET A Weekly Summary of U.S. COVID-19 Hospitalization Data, preliminary cumulative rates. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html . Accessed: February 9, 2022.
- COVID-NET A Weekly Summary of U.S. COVID-19 Hospitalization Data, characteristics of COVID-19-associated hospitalizations. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html . Accessed: February 9, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination . Accessed February 9, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths . Accessed: February 9, 2022.
- HHS Protect Public Data Hub. Washington, D.C.: US Department of Health and Human Services, 2022. https://protect-public.hhs.gov/pages/hospital-utilization . Accessed: February 3, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total . Accessed: February 9, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends . Accessed: February 9, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions . Accessed February 9, 2022.
- Omicron Variant: What You Need to Know. Coronavirus Disease 2019 (COVID-19) | COVID-19 | CDC Accessed: February 9, 2022.
Benefits and harms:
- Centers for Disease Control and Prevention (CDC). Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Moderna COVID-19 Vaccine. 2022. www.cdc.gov/acip/grade/bla-covid-19-moderna-vaccine.html
- Oliver, S. Evidence to Recommendation Framework: Moderna COVID-19 vaccine, Spikevax. Presentation to ACIP. February 4, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-04/07-COVID-Oliver-508.pdf
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#vaccine-confidence . Accessed January 21, 2022.
- Axios/Ipsos Poll. January 2022. https://www.ipsos.com/en-us/news-polls/axios-ipsos-coronavirus-index . Accessed January 19, 2022
- KFF COVID-19 Vaccine Monitor: Early Omicron Update (Dec 15 – 20, 2021). https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-early-omicron-update/ . Accessed January 19, 2022
- ABC news. August 31, 2021. More Americans getting vaccinated following full FDA approval of Pfizer COVID vaccine. https://abcnews.go.com/Health/americans-vaccinated-full-fda-approval-pfizer-covid-vaccine/story?id=79750505
- CDC and University of Iowa/RAND survey, unpublished
Acceptability:
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total . Data as February 9, 2022.
Feasibility:
- Moderna. Storage & Handling. https://eua.modernatx.com/covid19vaccine-eua/providers/storage-handling . Accessed January 18, 2022.
Resource use:
- Peterson-KFF Health System Tracker. December 22, 2021. Unvaccinated COVID-19 hospitalizations cost billions of dollars. https://www.healthsystemtracker.org/brief/unvaccinated-covid-patients-cost-the-u-s-health-system-billions-of-dollars/
- CDC COVID-19. COVID-19 Vaccines Are Free to the Public. www.cdc.gov/coronavirus/2019-ncov/vaccines/expect.html . Updated November 3, 2021.
- Padula WV, Malaviya S, Reid NM, et al. Economic value of vaccines to address the COVID-19 pandemic: a U.S. cost-effectiveness and budget impact analysis. J Med Econ. 2021 Jan-Dec;24(1):1060-1069. doi: 10.1080/13696998.2021.1965732.
- Bartsch SM, Ferguson MC, McKinnell JA. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020:39:927-35. DOI: 10.1377/hlthaff.2020.00426.
- Gupta S, Cantor J, Simon KI, et al. Vaccinations Against COVID-19 May Have Averted Up To 140,000 Deaths In The United States. Health Aff . 2021 Sep;40(9):1465-1472. doi: 10.1377/hlthaff.2021.00619.
- Kohli M, Maschio M, Becker D, Weinstein M. The Potential Public Health and Economic Value of a Hypothetical COVID-19 Vaccine in the United States: Use of Cost-Effectiveness Modeling to Inform Vaccination Prioritization. Vaccine 2021 Feb 12; 39(7): 1157–1164.
- COVID-NET Laboratory-confirmed COVID-19 hospitalizations. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalization-network . Accessed February 3, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends . Accessed February 3, 2022.
- CDC COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://covid.cdc.gov/covid-data-tracker/#county-view?list_select_state=all_states&list_select_county=all_counties&data-type=Vaccinations&metric=Administered_Dose1_Pop_Pct . Accessed February 3, 2022.
- KFF COVID-19 Vaccine Monitor: Differences in Vaccine Attitudes Between Rural, Suburban and Urban Areas. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-vaccine-attitudes-rural-suburban-urban/ . Accessed January 18, 2022.
- McNaghten A, Brewer NT, Hung M, et al. COVID-19 Vaccination Coverage and Vaccine Confidence by Sexual Orientation and Gender Identity — United States, August 29–October 30, 2021. MMWR Morb Mortal Wkly Rep 2022;71:171–176. DOI: http://dx.doi.org/10.15585/mmwr.mm7105a3
View the complete list of EtR Frameworks
ACIP comprises medical and public health experts who develop recommendations on the use of vaccines in the civilian population of the United States.
- Introduction
- Conclusions
- Article Information
A to D, Risk ratios (RRs) and 95% CIs for acute myocardial infarction (A), pulmonary embolism (B), myocarditis or pericarditis (C), and hemorrhagic stroke (D) were estimated using generalized linear models with a binominal distribution and log link function. Model 1 was unadjusted; model 2 was adjusted for region and month of vaccination; model 3 was adjusted for age, sex, race and ethnicity, and frailty; and model 4 was adjusted for region, month of vaccination, age, sex, race and ethnicity, frailty, claim source (eg, pharmacy and/or Medicare), time since prior documented COVID-19 infection, time since prior hospitalization, time since prior outpatient visit, and time since prior emergency department visit. Risk ratios are interpreted as the relative difference in the outcome between mRNA-1273 vs BNT162b2, whereby an RR of 1.00 represents no relative difference in risk.
A to D, Risk ratios (RRs) and 95% CIs for facial nerve palsy (A), thrombocytopenia purpura (B), pulmonary embolism (C), and myocarditis or pericarditis (D) were estimated using generalized linear models with a binominal distribution and log link function. Interaction terms between frailty subgroup and vaccine type were included to obtain stratum-specific estimates and to formally test for modification (interaction term: P < .05). Model 1 was unadjusted; model 4 was adjusted for region, month of vaccination, age, sex, race and ethnicity, claim source (eg, pharmacy and/or Medicare), time since prior documented COVID-19 infection, time since prior hospitalization, time since prior outpatient visit, and time since prior emergency department visit. Risk ratios are interpreted as the relative difference in the outcome between mRNA-1273 vs BNT162b2 within each frailty subgroup, whereby an RR of 1.00 represents no relative difference in risk within that frailty subgroup. The nonfrail estimates for facial nerve palsy and prefrail estimates for thrombocytopenia purpura are the same due to rounding.
Odds ratios (ORs) and 95% CIs were estimated using multinomial logistic regression, comparing the risk of pulmonary embolism alone, diagnosed COVID-19 alone, or both pulmonary embolism and diagnosed COVID-19 over follow-up. Model 1 was unadjusted; model 4 was adjusted for region, month of vaccination, age, sex, race and ethnicity, claim source (eg, pharmacy and/or Medicare), time since prior documented COVID-19 infection, time since prior hospitalization, time since prior outpatient visit, and time since prior emergency department visit. Odds ratios are interpreted as the relative difference in the outcome between mRNA-1273 and BNT162b2, whereby an OR of 1.00 represents no relative difference.
eTable 1. ICD-10-CM Diagnosis Codes Used to Define the Serious Adverse Event Outcomes in This Study
eTable 2. Participant Loss to Follow-up in the 28 Days Following the Week of the First Dose of an mRNA Vaccine Against COVID-19 (December 2020 to July 2021)
eTable 3. Risk of Serious Adverse Events and Diagnosed COVID-19 in the 28 Days Following the Week of the First Dose of mRNA-1273 or BNT162b2 Vaccines Among Community-Dwelling Medicare Fee-for-Service Beneficiaries in the US (December 2020 to July 2021)
eTable 4. Unadjusted and Adjusted Relative Risk of Serious Adverse Events and Diagnosed COVID-19 in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries in the US (December 2020 to July 2021)
eTable 5. Unadjusted and Adjusted Relative Risk of Serious Adverse Events and Diagnosed COVID-19 in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries Categorized as Nonfrail in the US (December 2020 to July 2021)
eTable 6. Unadjusted and Adjusted Relative Risk of Serious Adverse Events and Diagnosed COVID-19 in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries Categorized as Prefrail in the US (December 2020 to July 2021)
eTable 7. Unadjusted and Adjusted Relative Risk of Serious Adverse Events and Diagnosed COVID-19 in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries Categorized as Frail in the US (December 2020 to July 2021)
eTable 8. Unadjusted and Adjusted Relative Risk of Serious Adverse Events in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries in the US Who Did Not Have a Recent History of the Outcome Being Assessed (December 2020 to July 2021)
eTable 9. Unadjusted and Adjusted Relative Risk of Serious Adverse Events in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries in the US Who Had a Recent History of the Outcome Being Assessed (December 2020 to July 2021)
eTable 10. Unadjusted and Adjusted Relative Risk of Serious Adverse Events in the 21 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries in the US (December 2020 to July 2021)
eFigure. Adjusted Survival Curves for Pulmonary Embolism and Composite Outcome of Thromboembolic Events in the 28 Days Following the Week of the First Dose of mRNA-1273 Compared to BNT162b2 Vaccines Among Community-Dwelling Medicare Beneficiaries in the US (December 2020 to July 2021)
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Harris DA , Hayes KN , Zullo AR, et al. Comparative Risks of Potential Adverse Events Following COVID-19 mRNA Vaccination Among Older US Adults. JAMA Netw Open. 2023;6(8):e2326852. doi:10.1001/jamanetworkopen.2023.26852
Manage citations:
© 2024
- Permissions
Comparative Risks of Potential Adverse Events Following COVID-19 mRNA Vaccination Among Older US Adults
- 1 Center for Gerontology and Healthcare Research, Brown University School of Public Health, Providence, Rhode Island
- 2 Department of Health Services, Policy, and Practice, Brown University School of Public Health, Providence, Rhode Island
- 3 Department of Epidemiology, Brown University School of Public Health, Providence, Rhode Island
- 4 Providence Medical Center Veterans Administration Research Service, Providence, Rhode Island
- 5 Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts
- 6 Division of Gerontology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts
- 7 CVS Health Clinical Trial Services, Bell, Pennsylvania
- 8 Division of Geriatrics and Palliative Medicine, Alpert Medical School of Brown University, Providence, Rhode Island
Question Are there safety differences between mRNA vaccines for COVID-19, and do those differences vary by frailty level?
Findings In this cohort study of 6 388 196 older US adults, a 4% lower risk of pulmonary embolism, a 2% lower risk of thromboembolic events, and a 14% lower risk of diagnosed COVID-19 were observed among those who received the mRNA-1273 vaccine compared with the BNT162b2 vaccine. Although both vaccines were safe across frailty subgroups, differences were generally greater in individuals without frailty.
Meaning These findings suggest that compared with BNT162b2, mRNA-1273 was associated with a lower risk of adverse events, possibly due to improved protection against COVID-19.
Importance Head-to-head safety comparisons of the mRNA vaccines for SARS-CoV-2 are needed for decision making; however, current evidence generalizes poorly to older adults, lacks sufficient adjustment, and inadequately captures events shortly after vaccination. Additionally, no studies to date have explored potential variation in comparative vaccine safety across subgroups with frailty or an increased risk of adverse events, information that would be useful for tailoring clinical decisions.
Objective To compare the risk of adverse events between mRNA vaccines for COVID-19 (mRNA-1273 and BNT162b2) overall, by frailty level, and by prior history of the adverse events of interest.
Design, Setting, and Participants This retrospective cohort study was conducted between December 11, 2020, and July 11, 2021, with 28 days of follow-up following the week of vaccination. A novel linked database of community pharmacy and Medicare claims data was used, representing more than 50% of the US Medicare population. Community-dwelling, fee-for-service beneficiaries aged 66 years or older who received mRNA-1273 vs BNT162b2 as their first COVID-19 vaccine were identified. Data analysis began on October 18, 2022.
Exposure Dose 1 of mRNA-1273 vs BNT162b2 vaccine.
Main Outcomes and Measures Twelve potential adverse events (eg, pulmonary embolism, thrombocytopenia purpura, and myocarditis) were assessed individually. Frailty was measured using a claims-based frailty index, with beneficiaries being categorized as nonfrail, prefrail, and frail. The risk of diagnosed COVID-19 was assessed as a secondary outcome. Generalized linear models estimated covariate-adjusted risk ratios (RRs) and risk differences (RDs) with 95% CIs.
Results This study included 6 388 196 eligible individuals who received the mRNA-1273 or BNT162b2 vaccine. Their mean (SD) age was 76.3 (7.5) years, 59.4% were women, and 86.5% were White. A total of 38.1% of individuals were categorized as prefrail and 6.0% as frail. The risk of all outcomes was low in both vaccine groups. In adjusted models, the mRNA-1273 vaccine was associated with a lower risk of pulmonary embolism (RR, 0.96 [95% CI, 0.93-1.00]; RD, 9 [95% CI, 1-16] events per 100 000 persons) and other adverse events in subgroup analyses (eg, 11.0% lower risk of thrombocytopenia purpura among individuals categorized as nonfrail). The mRNA-1273 vaccine was also associated with a lower risk of diagnosed COVID-19 (RR, 0.86 [95% CI, 0.83-0.87]), a benefit that was attenuated by frailty level (frail: RR, 0.94 [95% CI, 0.89-0.99]).
Conclusions and Relevance In this cohort study of older US adults, the mRNA-1273 vaccine was associated with a slightly lower risk of several adverse events compared with BNT162b2, possibly due to greater protection against COVID-19. Future research should seek to formally disentangle differences in vaccine safety and effectiveness and consider the role of frailty in assessments of COVID-19 vaccine performance.
As of January 2023, approximately 70% of the global population has received at least 1 COVID-19 vaccine. 1 , 2 The BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) messenger RNA (mRNA) vaccines are among the most widely used, 3 , 4 aligning with recommendations from public health authorities and evidence of their superior safety and efficacy relative to other products. 5 - 7 Although the risk of serious adverse events following mRNA vaccine administration is low, 8 , 9 evidence regarding their comparative safety remains limited.
Few studies have directly compared the risk of potential adverse events between mRNA vaccines, which differ in their manufacturing, administration, and immune response. 10 - 12 Existing head-to-head comparisons of BNT162b2 and mRNA-1273 have shown small yet potentially meaningful differences in the risk of several adverse events that can vary by age and sex. 13 , 14 However, current estimates generalize poorly to older adults and are derived from samples that are too small to capture rare events over a short and clinically relevant follow-up period. Further, no studies to date have assessed comparative vaccine safety within and across patient subgroups with increased frailty or history of the diagnoses identified as vaccine-associated adverse events—conditions likely to modify vaccine response and potentially contribute to differences in safety. 15 , 16
Importantly, several of the potential vaccine-associated adverse events are also sequelae of SARS-CoV-2. 8 , 13 , 17 - 19 As previously suggested, 13 a more effective vaccine may appear to be safer for some outcomes due to the enhanced and differential prevention of COVID-19. Because of the prevalence of SARS-CoV-2 at the time of early vaccination efforts and observed differences in mRNA vaccine effectiveness, 20 additional studies are needed to understand the extent to which differences in adverse events may be attributed to differential early effectiveness.
To inform public health recommendations and clinical decision making, we used a large population-based cohort of more than 6 million older adults to compare the risk of potential adverse events shortly after the first dose of mRNA-1273 and BNT162b2. We also assessed whether frailty and prior history of the conditions identified as potential vaccine-associated adverse events modified comparative vaccine associations.
We conducted a retrospective cohort study using customer data from 2 large national pharmacy companies linked to Medicare claims between December 11, 2020, and July 11, 2021. 21 We matched pharmacy customer prescription and vaccination data deterministically to the 100% Medicare enrollment files based on name, address, and date of birth. Approximately 95% of records were successfully matched, creating a cohort of more than 28 million individuals aged 65 years or older. Medicare Parts A and B were used to capture inpatient, outpatient, carrier, skilled nursing, and COVID-19 vaccine claims, and the Common Medicare Environment was used to measure sociodemographics and enrollment. The Minimum Data Set captured nursing home residence. The Brown University Institutional Review Board approved this study and waived informed consent because deidentified secondary data were used. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
The study population comprised community-dwelling Medicare fee-for-service (FFS) beneficiaries aged 66 years or older who received an mRNA vaccine as their first COVID-19 vaccine dose during the study period. The study index date (ie, time 0) at which follow-up began was defined as the start (ie, Sunday) of the week that individuals received their first vaccine dose. The study population was restricted to Medicare FFS beneficiaries to capture relevant covariate information derived from FFS claims.
As of the study index date, we sequentially excluded individuals who were aged younger than 66 years, resided in long-term care, were in the hospital, were not continuously enrolled in FFS Medicare for the previous 12 months, had a documented COVID-19 diagnosis in the prior 4 weeks, had invalid vaccine data (eg, indicated as receiving both mRNA vaccines), or were deceased ( Figure 1 ). We excluded individuals aged younger than 66 years to allow for a 1-year look-back window for covariates and to ensure continuous FFS enrollment, as Medicare eligibility begins at age 65 years for most people. Residents in long-term care were excluded due to differences in vaccination efforts in residential settings than in the community. Similar to related work, 13 individuals with recently diagnosed COVID-19 were excluded to capture those eligible for vaccination.
Our primary exposure comparison of interest was the receipt of an initial dose of mRNA-1273 vs BNT162b2. Since the per-protocol and intention-to-treat estimands are identical with a single-dose exposure, we estimated both estimands in this study. The week of individuals’ first vaccine dose and vaccine manufacturer were identified using Current Procedural Terminology codes (0011A for mRNA-1273 and 0001A for BNT162b2) in the Medicare Part B/Carrier File and pharmacy records. To study a vaccine-naive population, we chose to assess the risk of adverse events following the first dose of an mRNA vaccine.
Twelve serious adverse events identified by the US Food and Drug Administration as being potentially associated with mRNA vaccines were included as primary outcomes. 17 , 22 Outcomes were measured using International Classification of Diseases, Tenth Revision, Clinical Modification ( ICD-10-CM ) diagnosis codes from FFS claims in Medicare Parts A and B: acute myocardial infarction, facial nerve palsy (Bell palsy), deep vein thrombosis, disseminated intravascular coagulation, encephalomyelitis or encephalitis, Guillain-Barre syndrome, hemorrhagic stroke, thrombocytopenia purpura, myocarditis or pericarditis, nonhemorrhagic stroke, pulmonary embolism, and transverse myelitis (eTable 1 in Supplement 1 presents all outcome definitions). A composite outcome that comprised events related to thromboembolic mechanisms (acute myocardial infarction, deep vein thrombosis, hemorrhagic stroke, nonhemorrhagic stroke, or pulmonary embolism) was also assessed. All ICD-10-CM outcome definitions were based on prior work. 17 , 22
The start of follow-up for all individuals was the first day of the week (Sunday) during which the first vaccine dose was administered and continued until one of the following events: death, occurrence of an outcome (each assessed separately), or end of follow-up (28 days or July 17, 2021), whichever occurred first. Individuals with a recorded outcome on the index date who thus had 0 days of follow-up were excluded from the analysis of that outcome. We chose a 28-day follow-up period to capture adverse events most likely to be related to the vaccine.
As of the index date, we obtained sociodemographic characteristics (age, sex, geographic region, 23 self-reported race and ethnicity, 24 dual eligibility, and billing source of the vaccine claim [pharmacy, Medicare, or both sources]) for all individuals. Race and ethnicity was included as a covariate to account for potential differences in vaccine access and likelihood of vaccination and included American Indian or Alaska Native, Asian, Black, Hispanic, White, other (represents its own category derived from the Common Medicare Environment, and not the combination of several races and ethnicities), or unknown or missing. History of comorbidities within the past year was captured using FFS claims. 25 We measured the number of weeks since an individual’s last COVID-19 diagnosis (eTable 1 in Supplement 1 presents relevant diagnosis codes), and weeks since most recent prior hospitalization, outpatient visit, and emergency department visit (individuals without these events were categorized as having no prior encounter). A claims-based frailty index was derived using a 1-year look-back window, with individuals being categorized as nonfrail (<0.15), prefrail (≥0.15 to <0.25), or frail (≥0.25). 26 Finally, we measured zip code–level social deprivation using the American Community Survey. 27
We used standardized differences to evaluate covariate balance between the mRNA-1273 and BNT162b2 vaccine groups. 28 For the primary analysis, all individuals meeting the eligibility criteria at the index date were considered at risk for each outcome, even if they had experienced that outcome previously. Risk ratios (RRs) with 95% CIs were estimated using generalized linear models (binomial distribution and log link function). Covariates that were imbalanced (>10% standardized difference 28 ) and/or determined to be clinically relevant were included in a series of models. Adjustment was conducted in stages to show the relative impact of different covariates and increasing adjustment: unadjusted (model 1); region and month of vaccination (model 2); age, sex, race and ethnicity, and frailty (model 3); and models 2 and 3 plus vaccine billing source (eg, pharmacy vs Medicare), time since prior diagnosed COVID-19, and time since prior hospitalization, outpatient visit, and emergency department visit (model 4). For outcomes with a statistically significant association in model 4, population-averaged risk differences (RDs) and 95% CIs were derived from the estimated probabilities.
We assessed potential variation in the comparative risk of adverse events across frailty level and prior history of the outcome being assessed (eTable 1 in Supplement 1 ). Product terms (eg, frailty × vaccine) in models 1 and 4 provided a test of effect measure modification on the multiplicative scale and the derivation of estimates within subgroups.
First, since the vaccines were not randomly assigned and potential confounding bias was a concern, we examined the 28-day risk of hip and vertebral fractures as negative control outcomes. 29 Second, to account for differences in dosing schedule between the vaccines, all of the primary outcomes were assessed at 21 days. Third, to contextualize the extent to which potential differences in adverse events may be related to early vaccine effectiveness, we compared the 28-day risk of diagnosed COVID-19 as a secondary outcome. For statistically significant outcomes and known sequelae of SARS-CoV-2 (eg, pulmonary embolism 19 ), we used multinomial logistic regression to compare the risk of the adverse event alone, diagnosed COVID-19 alone, and the co-occurrence of the adverse event and diagnosed COVID-19.
To provide assurance that unintentional errors in the analysis were not responsible for any findings, the cohort creation and outcome measurements were coded independently and in duplicate. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc), and Stata, version 17 (StataCorp LLC). Statistical significance was defined as P < .05. Data analysis began on October 18, 2022.
We identified 6 388 196 eligible Medicare beneficiaries who received their first dose of an mRNA vaccine; slightly over half (n = 3 390 704) received BNT162b2 ( Table ). Their mean (SD) age was 76.3 (7.5) years; 59.4% were women and 40.6% were men. In terms of race and ethnicity, 0.2% of individuals self-identified as American Indian or Alaska Native, 2.3% as Asian, 5.3% as Black, 0.9% as Hispanic, 86.5% as White, and 2.1% as other race or ethnicity; these data were missing or unknown for 2.7%. Diabetes (24.3%), congestive heart failure (11.9%), and cancer (15.1%) were the most common comorbidities. More than one-third of individuals were categorized as prefrail (38.1%) or frail (6.0%). Loss to follow-up due to death was very rare across all outcomes (<1.0%; eTable 2 in Supplement 1 ).
We observed few differences in baseline characteristics between groups. However, on average, individuals who received BNT162b2 were older (aged ≥90 years: 7.2% vs 5.2%; standardized difference, 0.09), were more likely to be Black (5.8% vs 4.8%; standardized difference, 0.05), and were more likely to be categorized as frail (6.8% vs 5.1%; standardized difference, 0.07).
The risk of all adverse events was low, with each occurring in less than 1.0% of eligible individuals (eTable 3 in Supplement 1 ). Deep vein thrombosis and pulmonary embolism were the most frequently identified events, occurring in 0.27% and 0.23% of individuals, respectively. Disseminated intravascular coagulation (0.002%), encephalomyelitis (0.0004%), Guillain-Barre syndrome (0.0003%), and transverse myelitis (0.0002%) were very rare and were thus not examined in the adjusted and/or stratified analyses due to instability of the model estimates.
Across models 1 to 4, increasing adjustment attenuated the relative differences between the mRNA vaccines ( Figure 2 and eTable 4 in Supplement 1 ). In model 4, individuals who received mRNA-1273 had a 4.0% lower risk of pulmonary embolism and a 2.0% lower risk of the composite outcome of any thromboembolic-related event, representing 1 to 16 fewer cases of pulmonary embolism and 1 to 24 fewer thromboembolic-related adverse events per 100 000 individuals relative to BNT162b2 (pulmonary embolism: RR, 0.96 [95% CI, 0.93-1.00]; RD, 9 [95% CI, 1-16] per 100 000 individuals; composite outcome: RR, 0.98 [95% CI, 0.96-1.00]; RD, 12 [95% CI, 1-24] per 100 000 individuals). The risk of disseminated intravascular coagulation was higher among those who received mRNA-1273, but the outcome was rare and the results were not statistically significant (RR, 1.41 [95% CI, 0.95-2.10]).
The risk of all adverse events increased with greater frailty (eTables 5 to 7 in Supplement 1 ). An interaction between frailty and vaccine product was observed for facial nerve palsy and thrombocytopenia purpura, with mRNA-1273 showing a 14.0% and 11.0% lower risk of both outcomes among individuals categorized as nonfrail, respectively ( Figure 3 and eTables 5 to 7 in Supplement 1 ). A gradient across frailty was observed for several outcomes. For example, in individuals categorized as nonfrail, mRNA-1273 was associated with a 6.0% reduced risk of pulmonary embolism compared with BNT162b2 (RR, 0.94 [95% CI, 0.88-1.00]); this benefit was gradually attenuated in individuals categorized as prefrail (RR, 0.97 [95% CI, 0.93-1.01]) and frail (RR, 1.00 [95% CI, 0.92-1.08]).
The risk of each adverse event was greater among individuals who had a prior history of that condition (eTables 8 and 9 in Supplement 1 ). Individuals who received mRNA-1273 and had no history of deep vein thrombosis had a lower risk of incident deep vein thrombosis compared with those who received BNT162b2 (RR, 0.94 [95% CI, 0.89-1.00]; P = .02 for interaction).
Individuals vaccinated with mRNA-1273 had a lower risk of hip and vertebral fractures, the negative control outcomes, in the unadjusted models (RR, 0.85 [95% CI, 0.81-0.88]); however, full adjustment nullified this association (RR, 0.99 [95% CI, 0.95-1.02]), suggesting sufficient confounding control. Outcomes assessed at 21 days replicated the primary analysis (eTable 10 in Supplement 1 ), and the survival curves for pulmonary embolism and the composite outcome fully overlapped during the first week and began to separate at approximately day 10 and widened over time (eFigure in Supplement 1 ).
The mRNA-1273 vaccine was associated with a lower risk of diagnosed COVID-19 after full adjustment (RR, 0.86 [95% CI, 0.83-0.87]); this association was attenuated in individuals categorized as frail (RR, 0.94 [95% CI, 0.89-0.99]; P = .01 for interaction). In a multinomial model comparing the risk of pulmonary embolism alone, diagnosed COVID-19 alone, and the co-occurrence of pulmonary embolism and diagnosed COVID-19, mRNA-1273 was associated with a significantly lower risk of COVID-19 alone (odds ratio [OR], 0.85 [95% CI, 0.83-0.87]) and the co-occurrence of pulmonary embolism and COVID-19 (OR, 0.80 [95% CI, 0.67-0.97]), but not pulmonary embolism alone (OR, 0.97 [95% CI, 0.94-1.00]; P = .06) ( Figure 4 ).
We compared the risk of potential adverse events between the mRNA-1273 and BNT162b2 vaccines in a cohort of more than 6 million older US adults. We observed that the risk of adverse events was very low in both vaccine groups, and the vaccines did not differ in risk for most outcomes in the overall analysis. However, mRNA-1273 was associated with a lower risk of some adverse events, including pulmonary embolism, compared with BNT162b2. Notably, individuals who received mRNA-1273 also had a 14.0% lower risk of diagnosed COVID-19. Because pulmonary embolism is a sequela of COVID-19, 19 this and potentially other observed differences in adverse events may be the result of early vaccine effectiveness and differential mitigation of COVID-19. Some variation in the comparative risk of adverse events and diagnosed COVID-19 was observed across subgroups, with mRNA-1273 showing generally larger protective associations in individuals categorized as nonfrail.
To date, a small number of studies have directly compared the safety of the BNT162b2 and mRNA-1273 vaccines and accounted for important clinical differences between groups. 13 , 14 Among a cohort of US veterans, Dickerman et al 13 compared the risk of potential vaccine-associated adverse events over 38 weeks using electronic health record data and sought to account for differential effectiveness by censoring on SARS-CoV-2. Relative to BNT162b2, mRNA-1273 was associated with a reduced risk of several outcomes, including thromboembolic events, myocarditis or pericarditis, and acute myocardial infarction. However, the authors cautioned that differences in SARS-CoV-2 incidence could not be ruled out as a potential explanation of differences in adverse events. We also observed a lower risk of several adverse events among those who received mRNA-1273 vs BNT162b2 in a larger and diverse cohort, over shorter follow-up, with robust confounding control, and across clinical subgroups. We also observed that mRNA-1273 was associated with a reduced risk of diagnosed COVID-19. 20
Given the overlap in adverse events identified as potentially being associated with mRNA vaccines and those attributable to SARS-CoV-2, differences in safety outcomes between vaccines should be considered alongside early effectiveness. 13 , 19 Differences in adverse events between vaccines may reflect the benefits of vaccination with a more effective product due to superior protection against COVID-19 and its sequelae. Results from our sensitivity analysis support the hypothesis that differences in the risk of pulmonary embolism between the vaccines are related to differential early effectiveness. Regardless of the underlying mechanism, however, the comparative reduction in morbidity associated with mRNA-1273 is notable and may have real benefits at the population level. Nonetheless, studies confirming the extent to which differences in adverse events can be attributed to early effectiveness are needed.
Assessments of potential adverse events by frailty level and their prior history of occurrence reinforced the primary analysis and provide evidence of mRNA vaccine safety in real-world and more clinically vulnerable populations. These analyses also preliminarily favor attributing the observed differences in adverse events to early effectiveness rather than safety. The mRNA-1273 vaccine was associated with generally larger reductions in adverse events and diagnosed COVID-19 among individuals categorized as nonfrail. Because frailty is known to attenuate vaccine response, 15 the greater immunogenicity associated with mRNA-1273 may have been diminished in individuals categorized as frail, thereby reducing its degree of differential protection against COVID-19 and its sequelae. 12 , 30
First, despite our large sample, several outcomes were too rare to examine with precision. Second, residual confounding remains a possibility and the smaller effect sizes reported herein should be interpreted with some caution. Additionally, early perceptions regarding differences in vaccine performance may have contributed to the nonrandom selection or administration of BNT162b2 and mRNA-1273. However, we adjusted for many factors and the results from the negative control outcome analysis demonstrate robustness. Third, incomplete outcome ascertainment is possible; however, with the potential exception of myocarditis, 14 , 18 we do not anticipate that outcomes would be differentially captured between vaccine groups. Fourth, we cannot confirm whether the observed differences in adverse events are due to a vaccine safety signal or differential effectiveness. Fifth, our use of administrative claims without chart review makes it challenging to determine the timing of adverse events and the temporal sequencing of diagnosed COVID-19. Similarly, since our follow-up period began at the start of the week, it was possible for adverse events to occur prior to the true vaccination date; however, due to the severity of the outcomes assessed, we suspect this sequencing to be rare. Finally, we do not have data on the risks of adverse events under study in an unvaccinated comparator group.
In this cohort study of older US adults, the risk of adverse events following BNT162b2 and mRNA-1273 administration was low for both mRNA vaccines, affirming their safety overall and in patient subgroups at potentially increased risk of adverse events. Because the risk of adverse events following natural infection exceeds that of either mRNA vaccine, 8 , 18 vaccination with any available product should be prioritized. Nonetheless, mRNA-1273 was associated with a slightly lower risk of pulmonary embolism and other adverse events compared with BNT162b2. Because individuals who received mRNA-1273 also had a lower risk of diagnosed COVID-19, the reduced risk of adverse events in this vaccine group may represent the benefits of vaccination with a more effective product. Future research should seek to formally disentangle differences in vaccine safety and effectiveness and consider the role of frailty in assessments of COVID-19 vaccine performance.
Accepted for Publication: June 21, 2023.
Published: August 2, 2023. doi:10.1001/jamanetworkopen.2023.26852
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Harris DA et al. JAMA Network Open .
Corresponding Author: Daniel A. Harris, PhD, Center for Gerontology and Healthcare Research, Brown University School of Public Health, 121 S Main St, Ste 649, Providence, RI 02912 ( [email protected] ).
Author Contributions: Drs Harris and Hayes had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Harris, Hayes, Zullo, Mor, Deng, Djibo, Gravenstein.
Acquisition, analysis, or interpretation of data: Harris, Hayes, Zullo, Mor, Chachlani, McCarthy, Djibo, McMahill-Walraven, Gravenstein.
Drafting of the manuscript: Harris, Zullo, Deng.
Critical review of the manuscript for important intellectual content: Harris, Hayes, Zullo, Mor, Chachlani, McCarthy, Djibo, McMahill-Walraven, Gravenstein.
Statistical analysis: Harris, Hayes, Zullo, Chachlani, McCarthy, Gravenstein.
Obtained funding: Zullo.
Administrative, technical, or material support: Hayes, Mor, Deng, McCarthy, Djibo, McMahill-Walraven, Gravenstein.
Supervision: Harris, Hayes, Zullo, Mor, McMahill-Walraven, Gravenstein.
Conflict of Interest Disclosures: Dr Hayes reported receiving grants from the National Institute of Aging (NIA) during the conduct of the study. In addition, Dr Hayes reported receiving consulting fees from the Canadian Association of Drugs and Technologies in Health and grants from Sanofi, Genentech, and Insight Therapeutics outside the submitted work. Dr Zullo reported receiving grants from Sanofi paid directly to Brown University for collaborative research on the epidemiology of infections and vaccinations among nursing home residents outside the submitted work. Dr Djibo reported receiving salary support via contracts and grant funding from the US Food and Drug Administration (FDA) Sentinel initiative (Sentinel and Biologics Effectiveness and Safety [BEST] programs), the NIA Imbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials (IMPACT) Collaboratory, TherapeuticsMD, Academy of Managed Care Pharmacy (AMCP) Biologics and Biosimilars Collective Intelligence Collaborative (BBCIC), and Reagan-Udall Foundation Innovation in Medical Evidence and Development Surveillance (IMEDS) multisite research service agreements funded by AbbVie, Merck, Novartis, and Pfizer awarded to and administrated by CVS Health outside the submitted work. In addition, Dr Djibo reported owning CVS stock options. Dr McMahill-Walraven reported receiving salary support via contracts and grant funding from the FDA Sentinel initiative (Sentinel Program and BEST Programs), the NIA IMPACT Collaboratory, TherapeuticsMD, AMCP Biologics and BBCIC, and Reagan-Udall Foundation IMEDS multisite research service agreements funded by AbbVie, Merck, Novartis, and Pfizer awarded to and administrated by CVS Health outside the submitted work. Dr McMahill-Walraven also reported owning CVS stock options and receiving personal fees from Brown University during the conduct of the study. In addition, Dr McMahill-Walraven reported receiving consulting fees from Pfizer to monitor vaccine complications outside the submitted work. Dr Gravenstein reported receiving grants from Pfizer for a pneumococcal vaccine; and consulting fees from Pfizer, Moderna, Sanofi, Janssen, Pfizer, Seqirus, GlaxoSmithKline, and Novavax outside the submitted work. In addition, Dr Gravenstein reported receiving honoraria from Sanofi, Seqirus, and Janssen for presentations and participation on advisory boards for Janssen, Pfizer, Sanofi, ReViral, and Vaxart outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by the NIA of the National Institutes of Health under award number U54AG063546 (Dr Mor), which funds the NIA IMPACT Collaboratory. Supplemental funding was provided under grant numbers 3U54AG063546-S07 and 3U54AG063546-S08 (Dr Mor).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /
Coronavirus disease (COVID-19): Vaccine research and development
Reviewed and current on 10 August 2021.
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety.
Vaccines go through various phases of development and testing – there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease, which is called efficacy. All phases assess safety. The last phase, phase III, are usually conducted in a large number of people, often 10’s of thousands. After that, the vaccine needs to go through a review by the national regulatory authority, who will decide if the vaccine is safe and effective enough to be put on the market, and a policy committee, who will decide how the vaccine should be used.
In the past, vaccines have been developed through a series of consecutive steps that can take many years. Now, given the urgent need for COVID-19 vaccines, unprecedented financial investments and scientific collaborations are changing how vaccines are developed. This means that some of the steps in the research and development process have been happening in parallel, while still maintaining strict clinical and safety standards. For example, some clinical trials are evaluating multiple vaccines at the same time. It is the scale of the financial and political commitments to the development of a vaccine that has allowed this accelerated development to take place. However, this does not make the studies any less rigorous.
The more vaccines in development the more opportunities there are for success.
Any longer-term safety assessment will be conducted through continued follow up of the clinical trial participants, as well as through specific studies and general pharmacovigilance of those being vaccinated in the roll out. This represents standard practise for all newly authorized vaccines.
In a regular vaccine study, one group of volunteers at risk for a disease is given an experimental vaccine, and another group is not; researchers monitor both groups over time and compare outcomes to see if the vaccine is safe and effective.
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
However, there are important ethical considerations that must be addressed – particularly for a new disease like COVID-19, which we do not yet fully understand and are still learning how to treat; it may be difficult for the medical community and potential volunteers to properly estimate the potential risks of participating in a COVID-19 human challenge study. For more information, see this WHO publication on the ethics of COVID-19 human challenge studies .
Small (phase I) safety studies of COVID-19 vaccines should enroll healthy adult volunteers. Larger (phase II and III) studies should include volunteers that reflect the populations for whom the vaccines are intended. This means enrolling people from diverse geographic areas, racial and ethnic backgrounds, genders, and ages, as well as those with underlying health conditions that put them at higher risk for COVID-19. Including these groups in clinical trials is the only way to make sure that a vaccine will be safe and effective for everyone who needs it.
Opportunities to volunteer for a COVID-19 vaccine trial vary from country to country. If you are interested in volunteering, check with local health officials or research institutions or email [email protected] for more information about vaccine trials.
The push for a COVID-19 vaccine
Vaccines explained
Related Q&As:
Vaccines and immunization: What is vaccination?
Coronavirus disease (COVID-19): Vaccines
Coronavirus disease (COVID-19): COVID-19 Vaccine access and allocation
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Vaccines, Blood & Biologics
- Resources for You (Biologics)
- Industry (Biologics)
Information About the Updated COVID-19 Vaccines
The Fall respiratory virus season is here, and people may have questions about the safety and effectiveness of the updated COVID-19 vaccines. Inaccurate information about these vaccines, particularly the mRNA COVID-19 vaccines, continues to circulate and could result in vaccine hesitancy, which in turn could lead to lower uptake of vaccines that are associated with protecting people from some of the most serious risks of COVID-19.
Based on a thorough assessment of the entire manufacturing process and the totality of clinical data gathered since the initial authorizations and through the most recent updates, the FDA is highly confident in the safety, effectiveness and quality of the mRNA COVID-19 vaccines approved and authorized for use in the United States. Over the past four years, the mRNA vaccines have been associated with a dramatic reduction in the risk of death, hospitalization and serious illness from COVID-19. Information about all of the updated COVID-19 vaccines (2024-2025 formula) available in the United States, including fact sheets, package inserts and supporting documents, can be found here .
Vaccination continues to be the cornerstone of COVID-19 prevention. The updated COVID-19 vaccines meet the FDA’s rigorous, scientific standards for safety, effectiveness, and manufacturing quality. Given waning immunity of the population from previous exposure to the virus and from prior vaccination, we strongly encourage those who are eligible to consider receiving any one of the updated COVID-19 vaccines to provide better protection against currently circulating variants. Additionally, CDC recommends everyone ages 6 months and older receive a 2024-2025 COVID-19 vaccine.
If you have questions about receiving an updated COVID-19 vaccine, please do not hesitate to speak with your health care provider.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis
Affiliations.
- 1 Neurology, Oregon Health and Science University School of Medicine, Portland, USA.
- 2 Education, Generalitat de Catalunya, Barcelona, ESP.
- PMID: 38234925
- PMCID: PMC10792266
- DOI: 10.7759/cureus.50703
Cancer is a complex and dynamic disease. The "hallmarks of cancer" were proposed by Hanahan and Weinberg (2000) as a group of biological competencies that human cells attain as they progress from normalcy to neoplastic transformation. These competencies include self-sufficiency in proliferative signaling, insensitivity to growth-suppressive signals and immune surveillance, the ability to evade cell death, enabling replicative immortality, reprogramming energy metabolism, inducing angiogenesis, and activating tissue invasion and metastasis. Underlying these competencies are genome instability, which expedites their acquisition, and inflammation, which fosters their function(s). Additionally, cancer exhibits another dimension of complexity: a heterogeneous repertoire of infiltrating and resident host cells, secreted factors, and extracellular matrix, known as the tumor microenvironment, that through a dynamic and reciprocal relationship with cancer cells supports immortality, local invasion, and metastatic dissemination. This staggering intricacy calls for caution when advising all people with cancer (or a previous history of cancer) to receive the COVID-19 primary vaccine series plus additional booster doses. Moreover, because these patients were not included in the pivotal clinical trials, considerable uncertainty remains regarding vaccine efficacy, safety, and the risk of interactions with anticancer therapies, which could reduce the value and innocuity of either medical treatment. After reviewing the available literature, we are particularly concerned that certain COVID-19 vaccines may generate a pro-tumorigenic milieu (i.e., a specific environment that could lead to neoplastic transformation) that predisposes some (stable) oncologic patients and survivors to cancer progression, recurrence, and/or metastasis. This hypothesis is based on biological plausibility and fulfillment of the multi-hit hypothesis of oncogenesis (i.e., induction of lymphopenia and inflammation, downregulation of angiotensin-converting enzyme 2 (ACE2) expression, activation of oncogenic cascades, sequestration of tumor suppressor proteins, dysregulation of the RNA-G quadruplex-protein binding system, alteration of type I interferon responses, unsilencing of retrotransposable elements, etc.) together with growing evidence and safety reports filed to Vaccine Adverse Effects Report System (VAERS) suggesting that some cancer patients experienced disease exacerbation or recurrence following COVID-19 vaccination. In light of the above and because some of these concerns (i.e., alteration of oncogenic pathways, promotion of inflammatory cascades, and dysregulation of the renin-angiotensin system) also apply to cancer patients infected with SARS-CoV-2, we encourage the scientific and medical community to urgently evaluate the impact of both COVID-19 and COVID-19 vaccination on cancer biology and tumor registries, adjusting public health recommendations accordingly.
Keywords: cancer; covid-19; malignancy; metastasis; oncogenesis; recurrence; sars-cov-2; spike glycoprotein; vaccines.
Copyright © 2023, Valdes Angues et al.
PubMed Disclaimer
Conflict of interest statement
The authors have declared that no competing interests exist.
Figure 1. Cancer-promoting molecular mechanisms and pathways…
Figure 1. Cancer-promoting molecular mechanisms and pathways potentially mediated by SARS-CoV-2 and/or certain COVID-19 vaccines
Figure 2. Spike-mediated ACE2 downregulation and cell…
Figure 2. Spike-mediated ACE2 downregulation and cell signaling might promote cancer progression in COVID-19 patients…
- Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Meslé MM, Brown J, Mook P, et al. Euro Surveill. 2021;26 - PMC - PubMed
- Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Health Aff (Millwood) 2021;40:1465–1472. - PMC - PubMed
- Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Lancet Infect Dis. 2022;22:1293–1302. - PMC - PubMed
- Estimating deaths averted and cost per life saved by scaling up mRNA COVID-19 vaccination in low-income and lower-middle-income countries in the COVID-19 Omicron variant era: a modelling study. Savinkina A, Bilinski A, Fitzpatrick M, et al. BMJ Open. 2022;12:0. - PMC - PubMed
- Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. Steele MK, Couture A, Reed C, et al. JAMA Netw Open. 2022;5:0. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 September 2024
COVID-19 vaccine refusal is driven by deliberate ignorance and cognitive distortions
- Kamil Fuławka 1 ,
- Ralph Hertwig ORCID: orcid.org/0000-0002-9908-9556 1 &
- Thorsten Pachur 1 , 2
npj Vaccines volume 9 , Article number: 167 ( 2024 ) Cite this article
63 Altmetric
Metrics details
- Epidemiology
Vaccine hesitancy was a major challenge during the COVID-19 pandemic. A common but sometimes ineffective intervention to reduce vaccine hesitancy involves providing information on vaccine effectiveness, side effects, and related probabilities. Could biased processing of this information contribute to vaccine refusal? We examined the information inspection of 1200 U.S. participants with anti-vaccination, neutral, or pro-vaccination attitudes before they stated their willingness to accept eight different COVID-19 vaccines. All participants—particularly those who were anti-vaccination—frequently ignored some of the information. This deliberate ignorance, especially toward probabilities of extreme side effects, was a stronger predictor of vaccine refusal than typically investigated demographic variables. Computational modeling suggested that vaccine refusals among anti-vaccination participants were driven by ignoring even inspected information. In the neutral and pro-vaccination groups, vaccine refusal was driven by distorted processing of side effects and their probabilities. Our findings highlight the necessity for interventions tailored to individual information-processing tendencies.
Similar content being viewed by others
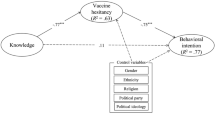
Misinformation of COVID-19 vaccines and vaccine hesitancy
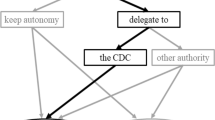
Information processing style and institutional trust as factors of COVID vaccine hesitancy
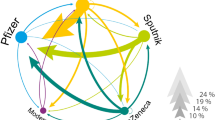
Understanding hesitancy with revealed preferences across COVID-19 vaccine types
Introduction.
In 2019, the World Health Organization listed vaccine hesitancy—the reluctance or refusal to get vaccinated despite the availability of vaccines—as one of the top 10 global health threats 1 . Vaccine hesitancy is a complex phenomenon determined by historical, political, and socio-cultural factors, as well as individual knowledge and risk perception 2 . Recent reviews of over 100 surveys in high-, middle-, and low-income countries indicate that concerns about the side effects (risks) and effectiveness (benefits) of COVID-19 vaccines are among the main reasons for vaccine hesitancy 3 , 4 . Accordingly, many interventions to reduce vaccine hesitancy aim at providing factual information on vaccine evidence —that is, possible harms, potential benefits, and their probabilities—in a comprehensible fashion (e.g., using fact boxes) 5 , 6 . However, there is evidence that such transparent communication of the evidence does not impact people’s vaccination intentions 7 . Moreover, qualitative investigations show that the decision to refuse vaccination can be driven by factors unrelated to vaccine evidence, such as experiences of racism and mistreatment by medical professionals 8 , distrust of the pharmaceutical industry, or alternative understandings of medicine 9 . This begs the question of how (if at all) people use information about vaccine evidence. Do they ignore it? If they process it, are there distortions in the cognitive processing? Could the information be processed differently by people with different vaccination attitudes? And how does the effect of possible cognitive distortions on vaccine refusal compare to the effect of other relevant factors, such as demographic variables?
In this article, we leverage theoretical and analytical ideas as well as methodological tools from cognitive and behavioral science that have been developed to study individual decision processes to investigate how individuals with different attitudes toward COVID-19 vaccines process information on vaccine evidence. Our approach, where people make accept–refuse vaccination decisions for various existing COVID-19 vaccines (similar to refs. 10 , 11 ), allows us to characterize and measure how people process commonly provided information about vaccine evidence; it also allows us to capture and compare the influence of extraneous factors (which are unrelated to vaccine-specific information) on people’s vaccination decisions. Previous studies based on surveys and descriptive analyses—showing that people are more willing to accept a vaccine when it is more effective and has fewer and less frequent side effects—have not been able to cast light on these details of the decision process 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 .
We used process-tracing methodology and computational modeling to examine the extent to which people may engage in deliberate ignorance 20 and how they may distort information on vaccine evidence during information processing. Figure 1 outlines our conceptual framework. In our study, we operationalize deliberate ignorance of vaccine evidence as choosing not to inspect a piece of information on a vaccine’s side effects, benefits, and their probabilities in the pre-decision phase. We distinguish three levels of deliberate ignorance: full, partial, and none. With full deliberate ignorance , people abstain from inspecting any information on vaccine evidence (Fig. 1 a); their decisions may then be based on other factors instead, such as trust in the government or the belief that COVID-19 is no worse than a common cold (see refs. 3 , 21 , 22 for other factors). With partial deliberate ignorance , people ignore some—but not all—of the vaccine evidence information. Here, we focus on a specific manifestation of partial deliberate ignorance, probability neglect in which a vaccination outcome (e.g., side effect) is inspected, but its probability is not (Fig. 1 b; see “Methods: Preregistration”). Probability neglect has been observed for dreadful risky outcomes 23 , 24 , including the side effects of medications 25 , 26 , 27 , 28 , 29 . These studies indicate that the neglected probability is treated as if the corresponding outcome was certain to occur, which, in case of outcomes such as vaccination side effects, would result in an increased rate of vaccine refusal (see Fig. 1 b). Finally, with no deliberate ignorance , people inspect all information on vaccine evidence and consider it in their decision (Fig. 1 c); even then, however, the cognitive processing of this information may be distorted (e.g., such that is it not fully considered in the decision) and thus deviate from what is considered a rational way to process information.

Different ways of processing information about the vaccine evidence, yielding an individual i 's probability of accepting vaccine v , denoted by P (accept). Path a represents full deliberate ignorance. Vaccine evidence is not inspected at all, and the decision is based on other factors related to the individual i and the vaccine v , denoted by β i and β v , respectively. Path b represents probability neglect, a type of partial deliberate ignorance in which only the possible outcomes of a vaccine but not their probabilities are acquired. In such cases, people usually perceive the outcome as certain to happen. However, in principle, it is also possible to ignore probability value and perceive the corresponding outcome as impossible to occur. Path c represents no deliberate ignorance. All information is inspected, but the probability information may be cognitively distorted via nonlinear probability weighting; the curvature of the probability weighting function measures the extent of such distortion. In paths ( b ) to ( c ), the neglected and weighted probabilities w ( p ) are integrated with the subjective values of the corresponding outcomes, which in the model are numerically represented by affect ratings a , transformed with value function v . The difference between the slopes of the value function over the side effects and the benefits of a vaccine constitutes a measure of loss aversion—a second cognitive distortion considered in our investigation.
Vaccination decisions can be conceptualized as instances of risky choice 30 , 31 . Based on research into risky choice, our conceptual framework considers two types of cognitive distortions: (nonlinear) probability weighting and loss aversion. Probability weighting refers to the observation that people make risky decisions as if they processed probabilities nonlinearly, with low and high probabilities being over- and underweighted, respectively 32 . In vaccination decisions, this would mean overweighting the typically low probabilities of side effects and underweighting the typically high probabilities of benefits of a vaccine (Fig. 1 , reduced probability sensitivity—stronger curvature of the probability weighting function indicates lower sensitivity). Loss aversion refers to the observation that people make risky decisions as if the psychological impact of losses is greater than that of gains 32 . In the context of vaccination decisions, loss aversion would mean that the psychological impact of possible side effects outweighs that of the potential benefits 33 , 34 , 35 . To capture loss aversion, comparable quantitative measures of the representations of the side effects and benefits are necessary. Medical outcomes often trigger pronounced self-reported 27 , 36 , 37 , 38 and physiological affective reactions 27 ; we therefore used positive and negative self-reported affect ratings of vaccines’ benefits and risks, respectively, to quantify people’s valuations of the outcomes. Loss aversion can then be measured by comparing the slopes of the value function over the side effects and the benefits of a vaccine, with a steeper slope over the side effects indicating loss aversion (Fig. 1 ).
To examine people’s information processing of vaccine evidence underlying COVID-19 vaccination decisions, we conducted an online study with U.S. citizens ( N = 1200) who self-reported as having anti- ( n = 365), neutral ( n = 373), or pro- ( n = 462) COVID-19 vaccine attitudes (vaccination attitude was measured using a single question with three response options; see “Methods: Study sample”). We recruited similarly large samples of anti-, neutral, and pro-vaccination participants to have comparable power when testing for potential processing differences between the three attitude groups (see “Methods: Preregistration”). Participants made a series of decisions about their willingness to get vaccinated with each of eight internationally licensed COVID-19 vaccines, one after the other. For each vaccine, participants could choose to inspect information on vaccine evidence, including side effects (e.g., blood clots, severe headache, tiredness) and benefits (protection against COVID-19 infection, severe illness, and death and the corresponding probabilities) (see Table 1 ). We recorded participants’ information inspection behavior—choosing to view a piece of information—with the process-tracing methodology Mouselab 39 (see “Methods: Mouselab task”). In Mouselab, the attributes of objects—here, pieces of information on vaccine evidence—are hidden behind labeled boxes, and each attribute can be inspected, one at a time, by hovering the mouse cursor over the respective box (Fig. 2 a). In each trial, the brand, vaccine technology, and country of development were clearly displayed at the top of the screen. Participants could decide based on this information without inspecting any information about the vaccine evidence. Participants could explore the available information as long and often as they wished before deciding whether to accept or refuse the vaccine. Finally, we obtained quantitative measures of each participant’s subjective valuations of the outcomes by asking them to provide affect ratings for each side effect and benefit (Fig. 2 b; see also “Methods: Affect rating task”).

a Example screen from the decision task. Only one information box could be inspected (uncovered) at a time by hovering the mouse cursor over it. b Example items from the affect rating task. Participants rated their negative affect for the 15 side effects in a Likert matrix table: All side effects were presented at once, making it easier for participants to provide internally consistent ratings. Ratings of positive affect for the three benefits of vaccination were collected on a separate screen. Note that the labels for the different levels of the rating scales were identical for positive and negative affect. In the analyses, the positive and negative affect ratings were coded as 1 to 5 and −5 to −1, respectively.
In all statistical analyses, we used Bayesian hierarchical models with participant-level intercepts—accounting for each participant making eight decisions—and the analyses were always conducted on the level of a single decision (see “Methods: Statistical modeling”). Our statistical inferences were based on the posterior distributions of the regression weight of interest (e.g., the difference between attitude groups in vaccine acceptance rates). For each dependent variable, we present the model-based predicted value of the variable (e.g., the posterior predictive distribution of vaccine acceptance probability) and the raw data (e.g., the observed vaccine acceptance proportions). The predicted values shown in the figures are medians of the posterior predictive distributions with 95% highest density intervals (HDI).
Vaccination decisions
A total of 61.9% (226/365) of participants in the anti-vaccination group, 11.7% (44/373) of participants in the neutral group, and 0.4% (2/462) of of participants in the pro-vaccination group refused all eight vaccines. On average, participants in the anti-vaccination, neutral, and pro-vaccination groups accepted one, three, and five of the eight vaccines, respectively. Interestingly, the non-zero acceptance rate in the anti-vaccination group was mainly driven by almost 30% of these participants indicating a willingness to accept the Indian vaccine Bharat Biotech.
We first tested how demographic and individual variables were related to the decision to accept or refuse a vaccine (Fig. 3 ). The strongest predictors of vaccine acceptance were vaccination attitude, the number of vaccinations against COVID-19 a participant had received by the time of the study, and vaccine brand. The raw data showed that political orientation and education level were related to vaccination decisions—consistent with results in previous studies (see ref. 3 ). These relationships, however, vanished when tested in the full statistical model, because political orientation and education (and age) had strong relationships with vaccination attitudes and the history of vaccinations against COVID-19 (see Supplementary Information ). The strong link between participants’ vaccination decisions measured in our study and their vaccination attitudes and actual vaccination history suggests that they took the decision task seriously.

The × symbols show empirical data; the + symbols show the posterior predictive acceptance probabilities from a Bayesian hierarchical logistic regression containing all presented variables. The horizontal line represents the median of the posterior predictive distribution, and the vertical line represents the 95% highest density interval.
We conducted two main sets of analyses to investigate the information processing underlying participants’ vaccination decisions. First, we used statistical models to investigate the relationship between deliberate ignorance of vaccine evidence (measured with Mouselab) and vaccination decisions. Second, we used computational modeling to investigate the cognitive distortions in the processing of the inspected vaccine evidence—that is, probability weighting and loss aversion—and its impact on vaccination decisions.
Deliberate ignorance and vaccination decisions
Our first main set of analyses focused on how participants’ vaccination decisions were related to whether they inspected all, some, or no information on vaccine evidence. We begin by analyzing full and partial deliberate ignorance and then examine probability neglect (a specific case of partial deliberate ignorance) in greater detail (see “Methods: Preregistration”).
In the next two sections, we report results from statistical models that included demographics and individual variables (Fig. 3 ) as covariates. In the Supplementary Information , we report robustness checks testing whether the inclusion of these covariates or the selection of specific subsets of covariates affects our conclusions. We note in the main text which results were not robust.
Did participants engage in deliberate ignorance, and how was it related to vaccination decisions?
Drawing on the data recorded with Mouselab, we analyzed participants’ information inspection behavior by assigning each trial to one of three levels of deliberate ignorance (see “Methods: Preprocessing of information inspection data”): (1) full deliberate ignorance—the vaccine evidence was not inspected at all, (2) partial deliberate ignorance—some but not all of vaccine evidence information was inspected, and (3) no deliberate ignorance—each piece of information on vaccine evidence was inspected at least once.
As shown in Fig. 4 a, anti-vaccination, neutral, and pro-vaccination participants exhibited full deliberate ignorance in 18%, 9%, and 7% of decisions, respectively. A comparison of the proportions of decisions with different levels of deliberate ignorance across the three participant groups showed that anti-vaccination participants ignored information to a larger extent than neutral (Δ = −1.03, 95% HDI: [−1.56, −0.51]) or pro-vaccination participants (Δ = −1.45, 95% HDI: [−2.19, −0.77]); the proportions did not differ between the neutral and pro-vaccination groups (Δ = −0.43, 95% HDI: [−0.16, 1]).

a Proportions of vaccination decisions preceded by different levels of deliberate ignorance, separately for the three attitude groups. Pie charts show the raw data; values in brackets are medians of the posterior predictive distributions from Bayesian hierarchical ordered-logit regression. b – e Relationships between the levels of deliberate ignorance and vaccine acceptance in the entire data set (panel b ) and separately for the anti-vaccination, neutral, and pro-vaccination participants. The × symbols show empirical data. The + symbols indicate the posterior predictive acceptance probabilities from Bayesian hierarchical logistic regressions—the horizontal line represents the median of the posterior predictive distribution, and the vertical line represents the 95% highest density interval. A triangle indicates when the 95% highest density interval of the posterior difference between the neighboring estimates excluded zero.
The level of deliberate ignorance was strongly related to vaccination decisions: The probability of vaccine refusal was highest when participants exhibited full deliberate ignorance and lowest when participants exhibited no deliberate ignorance (Fig. 4 b). This aggregate pattern also held within the anti-vaccination and neutral groups (Fig. 4 c–d). In the anti-vaccination group, full deliberate ignorance was almost always followed by vaccine refusal; in the pro-vaccination group, by contrast, full deliberate ignorance was associated with a higher probability of vaccine acceptance than partial deliberate ignorance. This suggests that in the pro-vaccination group, full deliberate ignorance was often driven by general and external factors such as trust in science and institutions that—from the point of view of these participants—made the inspection of information about vaccine evidence superfluous.
How was probability neglect related to vaccine refusal?
We defined probability neglect as cases in which there was at least one instance where a participant inspected an outcome but not its probability (see “Methods: Preprocessing of information inspection data”). Thus, probability neglect was indexed with a binary variable on the level of each trial: Probability neglect (1) occurred or (2) did not occur before the decision was made. We created separate indices of probability neglect for side effects and for benefits; we also measured probability neglect for side effects depending on the severity of the side effect (i.e., extreme, severe, or mild; see “Methods: Vaccine evidence data”).
Participants in the anti-vaccination, neutral, and pro-vaccination groups exhibited probability neglect for side effects in 15%, 13%, and 9% of vaccination decisions, respectively, and for benefits in 8%, 6%, and 4% of decisions, respectively. Furthermore, 47%, 40%, and 31% of participants in the anti-vaccination, neutral, and pro-vaccination groups, respectively, exhibited probability neglect for side effects in at least one vaccination decision; for benefits, the figures were 33%, 29%, and 19%, respectively. Nominally, both probability neglect for side effects and probability neglect for benefits were more frequent in the neutral and anti-vaccination groups than in the pro-vaccination group (Fig. 5 a), but these differences were not robust (see Supplementary Information ).

a Occurrence of probability neglect in information inspection, analyzed separately for side effects and benefits. A triangle indicates when the 95% highest density interval of the posterior difference between the neighboring estimates excluded zero. b Vaccine acceptance rates as a function of the type of probability neglect. In ( a ) and ( b ), the × symbols show empirical data. The + symbols show posterior predictive probabilities from Bayesian hierarchical logistic regressions—the horizontal line represents the median of the posterior predictive distribution, and the vertical line represents the 95% highest density interval. c Odds ratio values from a Bayesian hierarchical logistic regression. All factors were coded with sum-to-zero contrasts, such that the coefficients indicate the deviation from the average vaccine acceptance rate. The coefficients for vaccination attitude and history, and vaccine brand represent the effects of vaccination attitude, vaccine brand, and the interaction of the two; vaccination history refers to the effect of the number of actual vaccinations against COVID-19. The area outside the dashed lines is a space of effect size values considered practically relevant by convention (i.e., \(| \log (OR)| \ge 0.2\) ). d Odds ratio values from Bayesian hierarchical logistic regressions, estimated separately for each attitude group. The odds ratios for the predictors deliberate ignorance and probability neglect in panels ( c ) and ( d ) are the ratios for vaccine refusal.
To examine how probability neglect for side effects and benefits was related to vaccination acceptance or refusal, we tested them as predictors of vaccination decisions. Probability neglect for side effects was linked with an increased probability of vaccine refusal (OR refuse = 1.22, 95% HDI: [1.09, 1.35]), as was probability neglect for benefits (OR refuse = 1.22, 95% HDI: [1.05, 1.42]), but the latter result was not robust (see Supplementary Information ).
The effect of probability neglect on vaccination decisions may also depend on the severity of the side effect 40 . We therefore distinguished whether the probability neglect occurred for an extreme, severe, or mild side effect or for a benefit. The analysis revealed two robust effects (Fig. 5 b). First, vaccine refusal was much more likely in trials where the probability of an extreme side effect was neglected (Fig. 5 b–d). For example, participants who learned that AstraZeneca could lead to blood clots but did not learn that the probability of this side effect is extremely low were much more likely to refuse the vaccine than those who inspected both pieces of information. Second, vaccine refusal was much less likely in trials where the probability of a mild side effect was neglected. For instance, participants who learned that Sinovac could lead to tiredness but did not learn that this side effect occurs frequently were much more likely to accept the vaccine than those who inspected both pieces of information. This result makes intuitive sense but is at odds with the existing literature, which has focused only on how probability neglect leads to the avoidance of potentially dangerous events 23 , 24 .
The effects of probability neglect for extreme and mild side effects, and the effect of full deliberate ignorance, were substantially larger than the effects of the demographic variables and were among the strongest effects in the regression model (Fig. 5 c). The three effects also held across vaccination attitude groups (Fig. 5 d) and more than 30 different model specifications (see Supplementary Information ). Thus, how participants inspected and ignored information about vaccine evidence seemed to be a key predictor of their decision to get vaccinated with a given vaccine or not.
Cognitive distortions of vaccine evidence
In the next set of analyses, we used computational modeling to investigate the cognitive distortion of information on the vaccine evidence that participants inspected. Recall that in order to have a quantitative measure of participants’ subjective valuation of a vaccine’s possible outcomes, we asked each participant to rate (see Fig. 2 b) the negative (positive) emotion they would feel due to each side effect (benefit). Here, we start by comparing the average affect ratings across the three attitude groups. We then used the individual affect ratings as numerical inputs in computational modeling to investigate how the inspected information on vaccine evidence, including probabilities, was processed.
Did affect ratings of vaccine outcomes vary by vaccination attitude?
The average affect ratings of the anti-vaccination, neutral, and pro-vaccination groups are presented in Fig. 6 (see “Methods: Statistical modeling”). The anti-vaccination group gave the most negative affect ratings for side effects and the least positive ratings for benefits. The pro-vaccination group gave the least negative affect ratings for side effects and the most positive ratings for benefits. The affect ratings of the neutral group fell in between. Differences in average affect ratings between groups were much more pronounced for benefits (i.e., positive affect ratings) than side effects (i.e., negative affect ratings). These results show that vaccination attitudes are themselves associated with different emotional reactions to the possible outcomes of a vaccine that are independent of the vaccine brand or other external factors. All groups rated severe side effects as less negative than extreme side effects and more negative than mild side effects; this shows that the affect ratings track the objective magnitudes of the side effects and suggests that they constitute a reasonable measure of the subjective values of the vaccination outcomes for use in the computational modeling.

The × symbols show empirical data. The + symbols show expected mean affect ratings from Bayesian hierarchical ordered-logit regressions, estimated separately for each outcome group—the horizontal line represents the median of the posterior predictive distribution, and the vertical line represents the 95% highest density interval. A triangle indicates when the 95% highest density interval of the posterior difference between the neighboring estimates excluded zero.
Nonlinear probability weighting and loss aversion in vaccine decisions
We now turn to how participants processed the information about vaccine evidence that they inspected before making a vaccination decision. To this end, we developed a computational model (see “Methods: Computational modeling”) that can capture all paths to reach a decision presented in Fig. 1 . In the model, the probability of individual i accepting a vaccine v , denoted by P (accept), is a function of three additive components:
The β i parameter is an individual-level decision bias and captures a participant’s general propensity to accept or refuse a vaccine, irrespective of the vaccine’s properties. The β v parameter captures effects that are specific to a given vaccine, such as country of origin or the underlying technology, and do not pertain to the vaccine evidence; these effects are assumed to be the same for all participants. Finally, the term φ V i , v represents a model-based estimate of participant i ’s subjective valuation of vaccine v . As shown in Fig. 1 b–c, this valuation is based on vaccine evidence information—the vaccine’s outcomes, numerically represented by the individual affect ratings, and their probabilities—and is derived using prospect theory 32 (see “Methods: Formal model specification”).
To what extent were the vaccination decisions driven by individual decision biases, vaccine-specific effects, and subjective distortion of vaccine evidence? The first row in Fig. 7 addresses this question. For the majority of the anti-vaccination group, there was a decision bias (Fig. 7 a) to refuse the vaccine that was so strong that the effects of the vaccine’s properties (Fig. 7 b) and valuations (Fig. 7 c) rarely pushed the probability of acceptance above 50%. In the neutral group, the distribution of the decision bias was clustered below zero (Fig. 7 a), indicating that these participants showed a weak a priori propensity to refuse a vaccine; nevertheless, their vaccination decisions were also driven by vaccine-specific effects (Fig. 7 b) and by consideration of vaccine evidence information (Fig. 7 c). Almost all participants in the pro-vaccination group showed a bias toward accepting the vaccine (Fig. 7 a), but the size of this bias was not as large as the refusal bias in the anti-vaccination group. Overall, the distributions of subjective valuations V i v of the vaccine evidence (Fig. 7 c) were comparable to the distributions of the individual decision biases. This indicates that the subjective valuations of the vaccine’s effectiveness, side effects, and probabilities drove the vaccination decisions, particularly among the neutral and pro-vaccination participants.
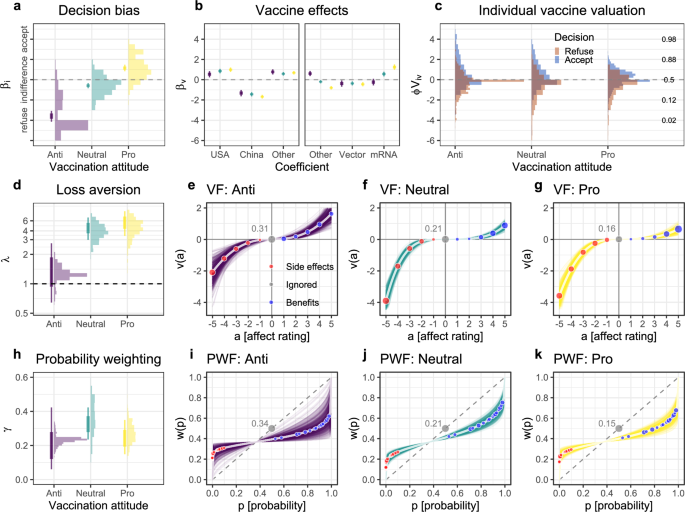
a Group-level (vertical lines) and individual-level (histograms) decision biases. b Group-level estimates of vaccine-specific effects on vaccination decisions. The coefficients show the predicted difference in the probability of refusing or accepting a vaccine from the average probability in a given group. c Distributions of subjective valuations φ V i , v , separately for accept and refuse decisions. The y -axis in panels ( a – c ) is on the scale of the linear predictor, which is then transformed via the inverse-logit function to obtain the predicted probability of vaccine acceptance shown on the right of panel ( c ). d Group-level (vertical lines) and individual-level (histograms) estimates of the loss aversion parameter λ . λ > 1 values indicate loss aversion; λ = 1 indicates loss neutrality. e – g Value function (VF) for the anti-vaccination, neutral, and pro-vaccination participants based on the group-level posterior distributions of the parameter estimates. h Group-level (vertical lines) and individual-level (histograms) estimates of the probability weighting parameter γ . Higher values of γ indicate more linear probability weighting. i – k Probability weighting function (PWF) for the anti-vaccination, neutral, and pro-vaccination participants based on the group-level posterior distributions of the parameter estimates. In panels ( a , d , and h ), the thin and thick vertical lines show 95% and 80% highest density intervals, respectively, of the posterior distribution of the group-level parameter estimates. The histograms show distributions of the posterior means of the individual-level parameters. The gray values and points in panels ( e – g ) and ( i–k ) give the proportions of ignored outcomes and probabilities, respectively, within each attitude group.
The value function captures the subjective valuation of the vaccines’ side effects and benefits (Fig. 7 d–g). These results show that nearly all participants exhibited substantial loss aversion. This is indicated by the individual-level values of the λ parameter above 1 (Fig. 7 d) and the asymmetric shapes of the curves over the ratings of side effects and benefits (Fig. 7 e–g). On average, the impact of a side effect rated as extremely negative was four times stronger than that of a benefit rated as extremely positive among the neutral and pro-vaccination participants. In the anti-vaccination group, the average degree of loss aversion was lower. Although this may seem counterintuitive, note that the anti-vaccination participants already expressed considerably stronger affective responses to the side effects than to the benefits (Fig. 6 ).
The probability weighting function (Fig. 7 h–k) captures the subjective valuation of probabilities, and the function’s shape is governed by the probability weighting parameter γ i , with higher values indicating more linear probability weighting (and lower values indicating lower sensitivity to differences in probability). On average, individual probability weighting γ i was highest in the neutral group and lowest in the anti-vaccination group. Additional analyses suggest that anti-vaccination participants ignored probabilities even after inspecting them (see Supplementary Information ). The inverse S-shaped form of the estimated probability weighting functions indicates that overall, the impact of small probabilities of side effects on vaccination decisions was higher than warranted based on their objective value; the impact of high probabilities of protection was smaller than warranted based on their objective value.
Qualitatively, both the value and probability weighting functions show relatively similar patterns across the three groups: All participants exhibited some degree of loss aversion and nonlinear probability weighting. Thus, computational modeling suggests that the vaccination decisions of all groups were characterized by some sort of aversion to side effects: Negative affect associated with side effects had more impact on vaccination decisions than did the positive affect associated with benefits (protection), probabilities of side effects were overweighted, and probabilities of benefits were underweighted. Since neutral and pro-vaccination participants were the most sensitive to vaccine evidence, these results suggest that cognitive distortion in the form of side-effect aversion was among the main reasons for vaccine refusal decisions in these two groups.
According to principles of good evidence communication 41 , the overarching aim should be to inform rather than persuade. This means, for instance, not cherry-picking findings and results but rather presenting “potential benefits and possible harms in the same way so that they can be compared fairly” (see p. 363 in ref. 41 . In light of these objectives of evidence communication, however, it is also crucial to understand the effects of evidence communication. For instance, how do people process evidence about the potential benefits and harms of vaccines that are provided to them? We used process-tracing methodology and computational modeling to investigate how participants with anti-vaccination, neutral, and pro-vaccination attitudes inspected and processed evidence about COVID-19 vaccines: Did they fully inspect all evidence or deliberately ignore some of it 20 ? And if they deviated from rational information processing, in what way?
All three attitude groups deliberately ignored some or all vaccine evidence information. Exhaustive inspection of the evidence was associated with higher vaccine acceptance. By contrast, inspecting information about possible extreme side effects but not their probabilities—an instance of probability neglect—was strongly associated with the decision to refuse a vaccine. Participants in all three groups valued the risks and benefits of vaccines unequally, showing aversion to side effects—in the sense that they had a stronger psychological response to the possible side effects of vaccines than to their potential benefits (akin to loss aversion in choices between risky prospects). In addition, all three groups overweighted the low probabilities of side effects, albeit to a different extent (see also refs. 33 , 35 ).
We also observed important differences between the groups in information processing. A substantial proportion of participants in the anti-vaccination group did not inspect any evidence about the vaccines. This full deliberate ignorance of vaccine evidence was almost always associated with vaccine refusal. Furthermore, the computational modeling analysis suggested that the anti-vaccination group’s high refusal rate was driven by a strong decision bias against vaccination. This means that in this group, the decision to refuse vaccination was essentially insensitive to evidence about the COVID-19 vaccines, even if evidence was initially inspected. There could be various reasons for this pronounced bias against vaccination, including mistrust in government, science, doctors, and health authorities 42 , 43 . Indeed, in the absence of basic trust, evidence about vaccines may be deemed to lack credibility. Participants with a vaccination-neutral attitude—who probably represent the largest group in the population 44 —displayed the most “rational” information processing. They showed the most linear probability weighting and overall highest sensitivity to vaccine evidence. Furthermore, they were not more likely to deliberately ignore vaccine evidence information than those in the pro-vaccination group.
Several insights follow from our findings. They underscore the importance of tailoring interventions to increase vaccine uptake to specific target groups. Our findings suggest a simple way to predict a person’s processing of information about vaccine evidence—namely, based on their attitude to COVID-19 vaccination. This can be measured by asking people to categorize themselves as pro-vaccination, neutral, or anti-vaccination. A person’s general vaccination attitude could be assessed before medical interviews or implemented on information websites. Based on the self-declared attitude, the content and format of the presented information could be adjusted accordingly (e.g., whether it is necessary first to establish trust or whether the focus should be on making risk information more accessible). However, this idea requires further testing, including how exactly people understand the ‘neutral’ vaccination attitude and how exactly to tailor the interventions to neutral and anti-vaccine participants. In the following paragraphs, we offer concrete ideas on how such tailored interventions could look like.
The prevalent deliberate ignorance of vaccine evidence among the anti-vaccination constitutes a practical barrier to the approach of risk communication that is meant to inform but not persuade 41 . Importantly, however, this does not mean that health communicators and health authorities tasked with evidence communication should abandon the goal of informing and instead give in to the urge to persuade or oversimplify (see ref. 41 ). Instead, it means that risk evidence communicators need to be realistic about their expectations. It also means that they must consider other aspects of their efforts, such as the relationship between the communicator and the audience (e.g., it may be advisable to deploy trusted community-based vaccination champions who are willing and able to engage in dialogue and support communication activities 45 ). In addition, it means explicitly addressing the major concerns of segments of the population that are skeptical of vaccination. This includes disclosing uncertainties and addressing what science does not know 41 , 46 ; communicating about side effects and adverse events in understandable, nontechnical, and transparent language to maintain trust and counter misinformation 5 , 47 ; and explicitly addressing vaccination-related myths and false information (see ref. 45 ).
Assuming that trust can be re-established among people with an anti-vaccination attitude, how will they process the evidence when they inspect it? Here, the results of our modeling approach are particularly relevant. In the anti-vaccination group, we observed that individuals, to the extent that they inspected the vaccine evidence, based their decisions solely on possible side effects and benefits (e.g., the protection against severe COVID-19) but disregarded the probabilities, even if they were inspected (for further details, see Supplementary Information ). This tendency to close one’s eyes to probabilities is qualitatively different from the information processing in the group of neutral participants. Based on these observations, targeted interventions that address this disregard of probabilities (including the overweighting of the side effects’ small probabilities) appear desirable.
What shape might such an intervention take? One approach is to use interactive simulations to convey vaccine evidence. Such simulations imitate the sequential and experiential mechanisms by which people naturally encounter risk information. A recent study 48 found that vaccination-hesitant adults were more likely to express an intention to get vaccinated when they learned about the likelihood of experiencing various COVID-related events with and without vaccination through interactive simulation relative to being presented with the same information in fact boxes. One possible reason for this effect may be the inescapable sampling involved in the interactive risk simulation. This may make it more difficult to ignore probability information (e.g., of side effects), leading to less deliberate ignorance and less distorted probability weighting. In addition, there is evidence that presenting probabilities in visual formats such as icon arrays, where icons represent people affected versus not affected, can reduce side-effect aversion 34 . Importantly, interactive simulations may also be a pertinent method to address the aversion to side effects in the group of people with neutral attitudes.
More generally, side-effect aversion occurred across all three groups. All groups valued possible risks higher than potential benefits (see Fig. 7 d–g). To avoid these strong reactions to side effects, authorities might be tempted not to disclose them or to disclose them selectively for fear of jeopardizing public vaccine acceptance. While such a policy may initially decrease vaccine hesitancy, it comes at a huge cost: Limited transparency undermines trust in health authorities and fosters the spread of conspiracy beliefs 47 . How is it possible to provide full transparency without prompting disproportionate overweighting of side effects or partial ignorance? One option is interactive simulations (described above); another is targeting the strong negative emotions associated with side effects. A recent multi-country study found that cognitive reappraisal (i.e., changing how one thinks about a situation) is an effective strategy for reducing negative emotions in the context of COVID-19 49 . By extension, cognitive reappraisal may also be effective in reducing negative emotions about vaccines. For example, the ‘rethinking’ strategy included in ref. 49 study could involve putting vaccination side effects into a broader perspective by emphasizing that any side effects would be less severe than COVID-19 symptoms without vaccination, or that extreme adverse events such as blood clots are in fact much more likely after a COVID-19 infection than after a vaccination—and that even when they do occur, the chances for successful treatment are very high.
We note a couple of limitations of our study. First, participants were paid a flat rate upon completion of the survey (see “Methods: Study sample”). This incentive scheme may have led some participants to click through the study as quickly as possible and collect the payment. However, given that Prolific participants have been shown to be of high quality 50 , 51 , and given our observation of systematic patterns of information inspection and processing across the three groups, it seems unlikely that such minimal-effort behavior critically shaped our results. Second, we did not explore people’s reasons for deliberate ignorance. Even though we instructed our participants to assume that the presented vaccine evidence is, in principle, applicable to them personally (see “Methods: Mouselab task”), some individuals may have engaged in deliberate ignorance because they thought of themselves as already fully informed about the evidence. If this was the case, however, participants should be more likely to ignore evidence related to the U.S.-approved vaccines but inspect evidence about the other, less familiar vaccines. However, we observed nearly as much deliberate ignorance with regard to vaccines approved for the U.S. as for those not approved: on average, 46% and 41%, respectively. Based on these numbers, it seems safe to conclude that preexisting knowledge of vaccine evidence was probably not among the main reasons for not inspecting the vaccine evidence in our investigation.
Relatedly, our results do not establish a causal link between deliberate ignorance and vaccination decisions but rather reveal associations between them. A widely discussed explanation for the public divide in beliefs about, for instance, vaccination, climate change, or evolution is that people engage in ‘motivated reasoning’ 52 . According to this argument, individuals skeptical about vaccination or climate change are inclined to reject ostensibly credible scientific information because it contradicts their prior beliefs. Rejecting scientific evidence to thus protect one’s beliefs (or even one’s identity) could take the form of not inspecting it altogether or inspecting only parts of it. It is possible that belief-protecting or identity-protecting processing underlies acts of deliberate ignorance (multiple causes have been suggested and discussed; see ref. 53 ). It is an exciting avenue for future research to investigate in more detail the reasons behind the information processing observed in the anti-vaccination group—such as motivated reasoning or suspicion of medical services caused by experiences of racial discrimination by public institutions. Finally, the strength of vaccine hesitancy is likely to vary across people. We have simplified its measurement so as to avoid complicating the complex modeling across groups. Future studies could use a more fine-grained measure of vaccine hesitancy, potentially providing further insights into the relationship between hesitancy and the processing of vaccine evidence.
Developing and manufacturing safe and effective COVID-19 vaccines within a year was a breathtaking success story of human ingenuity. Yet this story had a sobering aftermath: Worldwide, many people refused vaccination. It is now the task of behavioral scientists to understand the reasons for this phenomenon and to design better ways of communicating the available evidence on the risks and benefits of COVID-19 vaccines. Our findings that people often deliberately ignore vaccine evidence or process it in ways counter to rational standards suggest that effective evidence communication must take new and innovative paths. Societies can be fully prepared for future pandemics only when technological ingenuity is coupled with cognitive and behavioral insights.
Study sample
We used Prolific and its filtering options to collect complete data from 1200 U.S. adults and obtain a relatively balanced sample of participants with anti-vaccination, neutral, and pro-vaccination attitudes. We created three instances of the same study, each available to 400 participants. One was run with participants with anti-vaccination attitudes, another with participants with pro-vaccination attitudes, and a third with participants with neutral vaccine attitudes, as declared in the survey that Prolific provides to all platform users. At the end of the study, we asked participants about their vaccination attitudes to check for possible changes since the initial measurement by Prolific. Specifically, we asked “How would you characterize your general attitude toward vaccination against COVID-19? [Against, Neutral, Pro].” Most observed changes were in the pro-vaccination direction, and the final sample consisted of 365 anti-vaccination, 373 neutral, and 462 pro-vaccination participants. For all our analyses, we used the attitude that the participants reported at the end of the study.
The sample consisted of 720 women (60%), 463 men (39%), and 17 participants who chose “other” as their gender. The mean age was 38.23 years (SD = 13.76). We estimated that participants would need up to 20 minutes to complete the study; the actual median completion time was around 12 minutes. Participants were remunerated with 2.50 GBP. See Supplementary Fig. 1 for the distribution of all collected demographic variables within each attitude group.
The study was approved by the Internal Review Board (Ethics Committee) of the Max Planck Institute for Human Development. To participate in the study, each participant had to provide informed consent by accepting the terms and conditions outlined in the Study Information and Statement of Informed Consent for Adult Participants, presented at the beginning of the procedure. Data collection occurred between April 19 and April 25, 2022.
Vaccine selection
We included eight existing COVID-19 vaccines in the decision task, asking participants to state their willingness to accept or refuse vaccines (see Fig. 2 and “Methods: Mouselab task”). There were several reasons why we included more vaccines than only the three available in the U.S. First, we included multiple vaccines to be able to obtain a more precise measurement of individual tendencies to refuse or accept a vaccine. Second, the different vaccines differed in terms of their worst side effect and their effectiveness statistics (see Table 1 ); including these vaccines allowed us to get more precise estimates of the tested effects of probability neglect, loss aversion, and probability weighting. Third, we considered it relevant to include vaccines that are based on different technologies and/or were developed in different countries, as we wanted to measure to what extent participants’ decisions were sensitive to factors other than vaccination risks and benefits. Finally, including vaccines not available in the US reduced the possibility that people already had extensive knowledge of the vaccines’ risks and benefits.
Our specific inclusion criteria were as follows: We included all vaccines for which reliable clinical trial data of levels 3 and 4 were available in English at the time the study was designed. Initially, we also planned to include the Sputnik vaccine, but it was dropped when Russia launched the full-scale invasion of Ukraine.
Vaccine evidence data
The vaccine evidence information (i.e., vaccine effectiveness, side effects, and the corresponding probabilities) about the eight vaccines that were presented to the participants in the mouse lab choice is provided in Table 1 . A list of the data sources we used was sent to the participants after they had completed the study; the list can be obtained upon request. In general, we drew on phase 4 trials (i.e., data collected from monitoring the vaccine after releasing it to the public) for the data on vaccines’ effectiveness. If phase 4 trials were unavailable, we used results from phase 3 clinical trials (i.e., double-blind clinical trials involving thousands or tens of thousands of participants). The main sources for the side effects and their frequencies were also results from phase 3 trials or government reports. The latter were used mainly to obtain the frequencies of extremely rare side effects. We selected the three most severe side effects for each vaccine listed in the sources.
The eight selected vaccines included 15 side effects differing in severity: (1) Mild side effects: fever, tiredness, headache, and muscle pain; (2) Severe side effects: severe general discomfort, severe drowsiness, severe tiredness, severe headache, and severe muscle pain; (3) Extreme side effects: blood clots (thrombosis with thrombocytopenia syndrome), immune system attacking the nerves (Guillain-Barré syndrome) or the blood (immune thrombocytopenia), facial paralysis, heart muscle inflammation (myocarditis), and heart membrane inflammation (pericarditis).
Experimental design
All participants explicitly consented to the conditions of the study. The study had four main parts: (1) a Mouselab task, (2) a willingness-to-pay task implemented for exploratory analyses and not reported here, (3) an affect rating task, and (4) a post-experimental survey. Each task began with a brief introduction of its general purpose. The procedure was programmed using JavaScript and JSpsych by the first author and Maik Messerschmidt from the research IT support team of the Center for Adaptive Rationality at the Max Planck Institute for Human Development. The procedure, including the consent form, is available in a preview mode at https://covid-vax.exp.arc.mpib.org/ .
Mouselab task
Participants were presented with information on eight existing COVID-19 vaccines, one after the other, and were asked to indicate for each vaccine whether they would be willing to get vaccinated with it. The task started with a general introduction followed by detailed explanations of the task and the vaccine-related information, and a tutorial consisting of an example decision in which participants could try out how the information inspection boxes work. The vaccine-related information consisted of the developer/brand, country of origin, vaccine technology, risks (side effects and their frequencies), and benefits (effectiveness of the vaccine against COVID-19 infection, severe illness, and death due to the disease).
In each trial, the vaccine brand, country of origin, and vaccine technology were visible at the top of the screen. The outcomes of the vaccine (i.e., side effects and benefits) and their probabilities were hidden behind labeled black boxes; this information could be revealed by moving the mouse cursor over the box (Fig. 2 ). The information was visible as long as the cursor hovered over the box. Participants could freely explore the information as long and as often as they wished before making a decision, and the program recorded each hovering event. To approximate how vaccination risks and benefits tend to be presented in real life, the probabilities of side effects were presented as number of cases per 1,000,000 people, and effectiveness was presented using percentages that designated the relative risk reduction in the vaccinated population relative to the unvaccinated population. The presentation order of the vaccines, the relative position of risks to benefits, the relative positions of probabilities to outcomes, and the yes/no buttons were randomized between participants. However, to avoid confusion, the relative position of probabilities to outcomes and the yes/no buttons were held constant for each participant.
Before the actual task, participants were presented with a statement on the reliability of the presented data and were asked to “assume that the figures presented refer to the current wave of the pandemic and apply to you personally”: Please keep in mind that the figures provided for the vaccines were taken from official sources (vaccine package leaflets, clinical trial reports, government reports) and reflect the best current state of knowledge. However, as the pandemic evolves, these figures may change, especially as new variants emerge. In addition, data may vary across countries, age groups, and health conditions, and due to other factors. It is therefore possible that the figures presented here deviate from those you may have encountered in other contexts. This is unavoidable, but it does not mean that the figures presented here are incorrect. For the purpose of the study, please assume that the figures presented refer to the current wave of the pandemic and apply to you personally .
Affect rating task
This task consisted of two parts: affect ratings of the potential risks of the vaccines and affect ratings of the potential benefits. The presentation order of these parts was randomized between participants. Participants were asked to rate the overall negative and positive affect associated with each side effect and benefit.
The instruction for rating the side effects was as follows: In this task, you will be presented with a list of possible side effects. Your task is to imagine experiencing each of them after a COVID-19 vaccination. Please indicate the amount of negative emotion you would feel as a result of experiencing the event. We mean any negative emotion, such as feeling distressed, upset, guilty, ashamed, hostile, irritated, nervous, jittery, scared, or afraid .
Participants were then shown a Likert matrix table with 15 rows, each corresponding to one of the side effects (see Table 1 ).
The instruction for rating the vaccination benefits was as follows: In this task, you will be presented with a list of the negative outcomes that vaccines protect against. Your task is to imagine that you are fully protected from each outcome. Please assess the amount of positive emotion you would feel as a result of being protected from the event. We mean any positive emotion, such as feeling excited, enthusiastic, proud, determined, relieved, strong, or active .
Participants were then shown a Likert matrix table with three rows, each corresponding to one of the benefits (see Table 1 ).
The rating scale and example emotions listed in the instructions were based on the PANAS scale 54 . The labels for the negative and positive scales were identical. In both parts of this task, the order of the outcomes in the rating matrices was randomized for each participant.
Post-experimental survey
At the end of the study, we collected the following demographic information: sex (male, female, other), age in years (open-ended), racial identity (White, Black, Asian, multiracial, other), education (≤high school, some college education, Bachelor’s degree, ≥Master’s degree), political orientation (Democrat, Republican, Independent, other), and annual income (0–$30,000, $30,001–$60,000, $60,001–$99,999, ≥$100,000). Participants then reported the number of COVID-19 vaccinations they had received (open-ended), which vaccine they had received (BioNTech/Pfizer, Johnson & Johnson, Moderna), and how many times they had tested positive for COVID-19 (open-ended). We also asked: In your opinion, how likely is it that in the future you will (get COVID-19, get severe COVID-19, die from COVID-19). Participants answered this question using a rating scale with four options: definitely not, not likely, somewhat likely, and very likely. To measure vaccination attitude, we asked: How would you characterize your general attitude towards vaccination against COVID-19? (neutral, pro, against). The final question was open-ended: Were there specific reasons for how you searched for information about the vaccines in the decision task? How would you characterize your search behavior?
Preprocessing of information inspection data
An instance of information inspection was defined as an event during which a participant hovered a mouse cursor over a labeled black box (Fig. 2 a). Following standard practice, inspections that lasted less than 200 milliseconds were assumed to be incidental and removed from further analyses 55 . The remaining inspection data was used to construct trial-level (i.e., relating to a single vaccination decision) indices of deliberate ignorance for later usage in statistical and computational modeling. We based our analyses on the number of inspections of each piece of information rather than on total inspection times. The information we presented varied in format and character lengths (e.g., frequencies, percentages, text), which could affect inspection duration.
We distinguished between three levels of deliberate ignorance: full, partial, and none. The level of full deliberate ignorance was assigned to trials in the decision task in which no information on vaccine evidence was inspected. The level of partial deliberate ignorance was assigned to trials in which at least one information box on vaccine evidence was uncovered, excluding trials in which all information was inspected. The level of no deliberate ignorance was assigned to trials in which each piece of information on vaccine evidence was inspected at least once.
For all types of probability neglect investigated in the analyses (i.e., for benefits, side effects, and extreme, severe, and mild side effects), we distinguished between two levels: probability neglect either occurred or did not occur in the information inspection phase prior to the vaccination decision. A trial was classified as involving probability neglect of side effects if probability information for at least one side effect was not inspected, but the corresponding side effect was inspected; the same logic was used for benefits and for specific groups of side effects (i.e., mild, serve, or extreme).
Statistical modeling
All statistical models presented were estimated using the brms package 56 called from R 57 . All predictors were categorical and always coded with sum-to-zero contrasts. Posterior distributions of the models were estimated using four chains. Each chain consisted of 4000 iterations. The first half was used for burn-in, and only every second sample was recorded from the second half, resulting in 4000 recorded samples in total. The sampling procedure resulted in well-mixed chains, as indicated by \(\hat{R}\) values lower than 1.01.
We ran Bayesian hierarchical logistic regressions with a random intercept across participants for binary outcome variables (i.e., vaccine acceptance and probability neglect). For ordinal outcome variables (i.e., deliberate ignorance and affect ratings), we used Bayesian hierarchical ordinal regressions, developed specifically for these types of variables 58 . As priors for the regression coefficients in both types of models, we used zero-centered Student’s t-distribution, with a scale parameter of 2.5 and 3 degrees of freedom, which is considered a weakly informative prior (see: https://github.com/stan-dev/stan/wiki/Prior-Choice-Recommendations ).
The models were able to adequately capture the patterns in the data, as indicated by posterior predictive checks 59 . The approximated out-of-sample predictive performance of the reported statistical models varied from. 7 to. 87 (see Supplementary Information for more details on the evaluation of statistical models).
Predicted outcome values and pairwise comparisons
The posterior predicted outcome values presented in Figs. 3 – 6 were calculated using the conditional_effects function from the brms package used to estimate the models 56 . The predictions for a given predictor from a regression with multiple predictors were derived by setting all other predictor values to zero. Because all our models contained categorical predictors coded with sum-to-zero contrasts, these predictions are equivalent to taking the posterior of the global intercept from the model and adding it to the posteriors of the regression weights of a predictor of interest and passing the resulting values through the relevant link function (e.g., the inverse-logit function in the case of logistic regression).
To compute evidence for a difference between any two levels of a categorical predictor (reversed blue triangles in Figs. 4 – 6 ), we also drew on the posterior distributions of the regression weights. For a predictor with only two levels (e.g., attentional probability neglect: yes vs. no), we inferred that the data provided evidence for a difference in the outcome variable if the 95% HDI of the posterior distribution of the regression weight excluded zero.
For categorical predictors with more than two levels, the procedure for pairwise comparisons was more complex due to the sum-to-zero contrast factor coding used to estimate the models. First, for each factor level (e.g., vaccination attitude groups), the posterior predicted outcome value on the scale of the linear predictor was computed using the respective factor coding scheme and regression weights. Second, these posterior predicted outcome values were subtracted from each other to derive the posterior distribution of the outcome difference between any two levels of a factor of interest (e.g., anti-vaccination and neutral attitudes). Again, we inferred that the data provided evidence for a difference in the outcome variable between the two levels of a factor if the 95% HDI of the posterior distribution of the regression weight excluded zero.
Computational modeling
The model was written in the Stan programming language for statistical computing 60 . The posterior distribution of the model parameters was estimated using the rstan package 61 called from R 57 . The sampling procedure from the posterior distribution was based on four chains, each consisting of 2000 warm-up and 3000 subsequent samples. Every other sample was recorded, providing 6000 recorded samples in total. The sampling procedure resulted in autocorrelation-free and well-mixed chains, as indicated by \(\hat{R}\) values lower than 1.01.
The leave-one-out balanced predictive accuracy for the models reported in the main text was 0.81, 0.74, and 0.76 for the anti-vaccination, neutral, and pro-vaccination groups, respectively. The Supplementary Information provides additional analyses of model performance evaluation, including a comparison of the performance of alternative, simpler models.
Formal model specification
The probability of individual i accepting the vaccine v , denoted by P (accept), was given by
The β i parameter represents an individual-level decision bias: It indicates to what extent the participant i tends to accept or refuse a vaccine irrespective of the vaccine properties and evidence. The term X v β j consists of a 3 × 4 matrix of sum-to-zero contrasts X v and corresponding β j parameters. The first two columns of X v code the country of origin (United States, China, other); the next two columns code the vaccine technology (mRNA, vector, other). The term ϕ V i , v is the participant’s i subjective value of vaccine v determined based on prospect theory 32 . The V i , v component consists of the value function v ( a ), which takes participant i ’s affect ratings for side effects a i , s e and benefits a i , b as inputs, and of a probability weighting function w ( p ), which takes the probabilities of side effects p s e and benefits p b as inputs (with the latter technically being the effectiveness of the vaccine, see Supplementary Information ):
The value function v ( a ) has three cases, depending on whether the affect rating pertains to a side effect ( a i , s e ), a benefit ( a i , b ), or an outcome ignored in the information inspection pre-decision phase:
where the λ i ∈ [0, 1] parameter is a measure of loss aversion estimated separately for each participant i . With λ i = 0.5, both side effects and benefits are weighted equally, while values of λ i > 0.5 indicate an overweighting of side effects relative to benefits—which can be interpreted as loss aversion. Note that in most applications to monetary lotteries, the loss aversion parameter Λ is used as a multiplier of the negative consequences and estimated on the scale of positive real numbers. Our approach is algebraically equivalent since Λ = λ i /(1 − λ i ), but resulted in better model convergence.
The α > 0 parameter allows for a nonlinear transformation of the affect ratings. This may be necessary because the affect ratings were measured using an ordinal Likert scale (see Fig. 2 b), and the assumption of equal distances between the scale levels may not hold here (but the model allows for a linear mapping with α = 1). Note that when the α parameter is large, for example, when α = 3, the value of an extreme affect coded as a = 5 would be v (5) = λ × 125. Such large values would have a dominating effect within the logit function in Equation ( 2 ). For this reason, the V i , v component is scaled with the φ ∈ [0, 1] parameter—allowing any level of nonlinearity in the affect ratings scale but ensuring that the final value of the φ V i , v term is as large as supported by the data.
The function w ( p ) in Equation ( 2 ) transformed the probabilities of side effects and benefits into decision weights and had two cases, depending on whether the probability was inspected or neglected (i.e., deliberately ignored):
The first case in Equation ( 5 ) is an inverse S-shaped probability weighting function 62 that transforms objective probabilities into subjective decision weights. The free parameter γ i ∈ [0, 1] governs the curvature of the probability weighting function and is interpreted as probability sensitivity, with higher values indicating higher sensitivity. When γ i = 0, the function becomes a horizontal line with all decision weights w ( p ) = 0.37. When γ i = 1, the function indicates perfect probability sensitivity, that is, w ( p ) = p . The second case of Equation ( 5 ) applies in situations in which an outcome was inspected but its corresponding probability was not; in the main analyses, for these instances of probability neglect, we set w ( p ) = 0.5, which means that the decision-maker acknowledges the probabilistic nature of the inspected outcome. See the Supplementary Information for an extended discussion on the assumptions underlying the estimation of the weighting function, including the value of the neglected probability and interpretation of the vaccine effectiveness.
In sum, three parameters of the model were estimated on the individual level (i.e., for each participant): decision bias β i , loss aversion λ i , and probability sensitivity γ i . The parameters α and φ were only estimated on the group level because there were only eight data points (i.e., decisions) per participant, and we had no theoretical interest in estimating these parameters on the individual level.
Stan implementation and prior distributions
The individual-level parameters were modeled as a sum of a corresponding group-level parameter and individual-level displacements ζ i :
where the function Φ() is an approximation to the cumulative normal distribution function implemented in Stan and ensures that the resulting individual-level parameters are always in the required 0–1 range. The individual displacements are assumed to follow a multivariate normal distribution with mean μ = [0, 0, 0] and variance–covariance matrix Σ , also estimated from the data.
In terms of priors, we used standard normal distribution for the individual- and group-level decision biases β i and β , respectively, and also for the vaccine effects coefficients β v , thus assuming that the biases to accept or refuse a vaccine are equally likely.
We could also use the standard normal distribution as the priors for the group-level parameters on the probit scale: λ Φ , γ Φ , and φ Φ , which after transformation to the scale of actual parameter values via probit function Φ −1 () resulted in uniform priors on the 0–1 range—in line with the theoretical bounds of the parameters:
The α parameter was also modeled on the scale of real values and received a normal prior with a mean of zero and a standard deviation of 0.5. The parameter was then transformed via the exponential function to the scale of positive reals, resulting in a prior with a mode of one (i.e., linear usage of the Likert scale) and assuming that the plausible parameter values are in the 0–4 range.
Finally, to model the multivariate distribution of the individual displacements ζ i , we used weakly informative Lewandowski-Kurowicka-Joe (LKJ) prior with parameter η = 5 for the correlation matrix, which assumed that the most probable correlations between the individual parameters were in the range from −0.5 to 0.5. The prior for the standard deviation of the individual displacements \({\zeta }_{i}^{\beta }\) was the gamma distribution with shape and rates equal to 2 and 1, respectively, thus ensuring that the parameter is positive and likely in the 0–3 range. The priors for the standard deviations of the individual displacements \({\zeta }_{i}^{\lambda }\) and \({\zeta }_{i}^{\gamma }\) were normal distributions with a mean of 0.5 and standard deviation of 0.13, ensuring that the resulting standard deviation is within 0–1 range. This condition was necessary to avoid bimodal individual-level posterior distributions of the λ i and γ i parameters after the Φ() transformation in Equation ( 6 ).
Preregistration
The study design, including sample size and the number of vaccination decisions, was preregistered on April 19, 2022: https://aspredicted.org/66W_95Q . Four research questions were formulated, all relating to probability neglect. Specifically, we were interested in (1) whether people exhibit probability neglect in vaccination decisions, (2) whether probability neglect rates are associated with vaccination attitudes, (3) how probability neglect is associated with vaccination decisions; (4) to what extent probability neglect applies to side effects and benefits. All these research questions are addressed in the main text in the section “How was probability ignorance related to vaccine refusal?” using analytical methods specified in the preregistration. In the preregistration, we also considered weaker definitions of probability neglect (e.g., a lower number of acquisitions of probability information than of the corresponding outcome) than the one used in the paper. However, we decided to use the stricter definition of probability neglect here: acquisition of outcome but not probability information.
At the end of the preregistration, in the section titled Other , we also mentioned our plan to analyze the data using computational modeling and noted that to this end, we would collect affect ratings for side effects and benefits to use as numerical inputs in the models. Our modeling approach followed current best practices (see “Methods: Computational modeling”) and was based on prospect theory—a model previously used to analyze medical choices 25 , 26 , 27 , 28 , 29 , 63 . The model we developed deviated from the standard applications to simple monetary lotteries because vaccination decisions are more complex. Our model was developed to accurately capture patterns in the data and account for various factors driving vaccination decisions (see Supplementary Information ).
Data availability
All study data are available on the first author’s public GitHub repository: https://github.com/kfulawka/vax_info_neglect .
Code availability
The code needed to reproduce the analyses reported in the article is available on the first author’s public GitHub repository: https://github.com/kfulawka/vax_info_neglect .
World Health Organization. Ten Threats to Global Health in 2019 . https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (2019).
Dubé, E. et al. Vaccine hesitancy: an overview. Hum. Vaccin. Immunother. 9 , 1763–1773 (2013).
Article PubMed PubMed Central Google Scholar
Aw, J., Seng, J. J. B., Seah, S. S. Y. & Low, L. L. COVID-19 vaccine hesitancy—a scoping review of literature in high-income countries. Vaccines 9 , 900 (2021).
Article CAS PubMed PubMed Central Google Scholar
Solís Arce, J. S. et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 27 , 1385–1394 (2021).
World Health Organization. Vaccination and Trust: How Concerns Arise and the Role of Communication in Mitigating Crises . https://apps.who.int/iris/handle/10665/343299 (2017).
Lewandowsky, S. et al. The COVID-19 Vaccine Communication Handbook. A Practical Guide for Improving Vaccine Communication and Fighting Misinformation . https://www.movementdisorders.org/MDS-Files1/The_COVID-19_Vaccine_Communication_Handbook.pdf (2022).
Brick, C., McDowell, M. & Freeman, A. L. Risk communication in tables versus text: a registered report randomized trial on ‘fact boxes’. R. Soc. Open Sci. 7 , 190876 (2020).
Charles, N. Suspicion: Vaccines, Hesitancy, and the Affective Politics of Protection in Barbados (Duke Univ. Press, 2021).
Hausman, B. L. Anti/Vax: Reframing the Vaccination Controversy (ILR Press, 2019).
Kreps, S., Dasgupta, N., Brownstein, J. S., Hswen, Y. & Kriner, D. L. Public attitudes toward COVID-19 vaccination: the role of vaccine attributes, incentives, and misinformation. NPJ Vaccines 6 , 73 (2021).
Stöckli, S. et al. Which vaccine attributes foster vaccine uptake? a cross-country conjoint experiment. PLoS ONE 17 , e0266003 (2022).
Freeman, D. et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (OCEANS) II. Psychol. Med. 52 , 3127–3141 (2022).
Article PubMed Google Scholar
Marzo, R. R. et al. Perceived COVID-19 vaccine effectiveness, acceptance, and drivers of vaccination decision-making among the general adult population: a global survey of 20 countries. PLoS Negl. Trop. Dis. 16 , e0010103 (2022).
Prosser, L. A. et al. A discrete choice analysis comparing COVID-19 vaccination decisions for children and adults. JAMA Netw. Open 6 , e2253582 (2023).
Cerda, A. A. & García, L. Y. Hesitation and refusal factors in individuals’ decision-making processes regarding a coronavirus disease 2019 vaccination. Front. Public Health 9 , 626852 (2021).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl Acad. Sci. USA 118 , e2021726118 (2021).
Liu, H. et al. How information processing and risk/benefit perception affect COVID-19 vaccination intention of users in online health communities. Front. Public Health 11 , 1043485 (2023).
Strickland, J. C. et al. Behavioral economic methods to inform infectious disease response: prevention, testing, and vaccination in the COVID-19 pandemic. PLoS ONE 17 , e0258828 (2022).
Toro-Ascuy, D. et al. Factors influencing the acceptance of COVID-19 vaccines in a country with a high vaccination rate. Vaccines 10 , 681 (2022).
Hertwig, R. & Engel, C. Homo ignorans: deliberately choosing not to know. Perspect. Psychol. Sci. 11 , 359–372 (2016).
Jennings, W. et al. Lack of trust, conspiracy beliefs, and social media use predict COVID-19 vaccine hesitancy. Vaccines 9 , 593 (2021).
Robertson, E. et al. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 94 , 41–50 (2021).
Sunstein, C. R. Probability neglect: emotions, worst cases, and law. Yale Law J. 112 , 61–107 (2002).
Article Google Scholar
Sunstein, C. R. & Zeckhauser, R. Overreaction to fearsome risks. Environ. Resour. Econ. 48 , 435–449 (2011).
Pachur, T., Hertwig, R. & Wolkewitz, R. The affect gap in risky choice: affect-rich outcomes attenuate attention to probability information. Decision 1 , 64–78 (2014).
Pachur, T., Suter, R. S. & Hertwig, R. How the twain can meet: prospect theory and models of heuristics in risky choice. Cogn. Psychol. 93 , 44–73 (2017).
Suter, R. S., Pachur, T., Hertwig, R., Endestad, T. & Biele, G. The neural basis of risky choice with affective outcomes. PLoS ONE 10 , e0122475 (2015).
Lejarraga, T., Pachur, T., Frey, R. & Hertwig, R. Decisions from experience: from monetary to medical gambles. J. Behav. Decis. Mak. 29 , 67–77 (2016).
Suter, R. S., Pachur, T. & Hertwig, R. How affect shapes risky choice: distorted probability weighting versus probability neglect. J. Behav. Decis. Mak. 29 , 437–449 (2016).
Mousavi, S. & Gigerenzer, G. Risk, uncertainty, and heuristics. J. Bus. Res. 67 , 1671–1678 (2014).
Reyna, V. F., Broniatowski, D. A. & Edelson, S. M. Viruses, vaccines, and COVID-19: explaining and improving risky decision-making. J. Appl. Res. Mem. Cogn. 10 , 491–509 (2021).
Tversky, A. & Kahneman, D. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 5 , 297–323 (1992).
Waters, E. A., Weinstein, N. D., Colditz, G. A. & Emmons, K. M. Aversion to side effects in preventive medical treatment decisions. Br. J. Health Psychol. 12 , 383–401 (2007).
Waters, E. A., Weinstein, N. D., Colditz, G. A. & Emmons, K. M. Reducing aversion to side effects in preventive medical treatment decisions. J. Exp. Psychol. Appl. 13 , 11 (2007).
Waters, E. A., Weinstein, N. D., Colditz, G. A. & Emmons, K. Explanations for side effect aversion in preventive medical treatment decisions. Health Psychol. 28 , 201 (2009).
Loewenstein, G. Hot–cold empathy gaps and medical decision making. Health Psychol. 24 , S49–S56 (2005).
Izadi, S., Pachur, T., Wheeler, C., McGuire, J. & Waters, E. A. Spontaneous mental associations with the words “side effect”: implications for informed and shared decision making. Patient Educ. Couns. 100 , 1928–1933 (2017).
Waters, E. A., Pachur, T. & Colditz, G. A. Side effect perceptions and their impact on treatment decisions in women. Med. Decis. Mak. 37 , 193–203 (2017).
Payne, J. W., Bettman, J. R. & Johnson, E. J. The Adaptive Decision Maker (Cambridge Univ. Press, 1993).
Fuławka, K. & Pachur, T. An affective probability weighting function for risky choice with nonmonetary outcomes. In Proc. Annual Meeting of the Cognitive Science Society , Vol. 44 (eds Culbertson, J. et al.) 1025–1032. https://escholarship.org/uc/item/5bg7f816 (2022).
Blastland, M., Freeman, A. L., van der Linden, S., Marteau, T. M. & Spiegelhalter, D. Five rules for evidence communication. Nature 587 , 362–364 (2020).
Article CAS PubMed Google Scholar
Lindholt, M. F., Jørgensen, F., Bor, A. & Petersen, M. B. Public acceptance of COVID-19 vaccines: cross-national evidence on levels and individual-level predictors using observational data. BMJ Open 11 , e048172 (2021).
Viskupič, F., Wiltse, D. L. & Meyer, B. A. Trust in physicians and trust in government predict COVID-19 vaccine uptake. Soc. Sci. Q. 103 , 509–520 (2022).
Johnson, N. F. et al. The online competition between pro-and anti-vaccination views. Nature 582 , 230–233 (2020).
Betsch, C. et al. A call for immediate action to increase COVID-19 vaccination uptake to prepare for the third pandemic winter. Nat. Commun. 13 , 7511 (2022).
Wegwarth, O., Wagner, G. G., Spies, C. & Hertwig, R. Assessment of German public attitudes toward health communications with varying degrees of scientific uncertainty regarding COVID-19. JAMA Netw. Open 3 , e2032335 (2020).
Petersen, M. B., Bor, A., Jørgensen, F. & Lindholt, M. F. Transparent communication about negative features of COVID-19 vaccines decreases acceptance but increases trust. Proc. Natl Acad. Sci. USA 118 , e2024597118 (2021).
Wegwarth, O. et al. Vaccination intention following receipt of vaccine information through interactive simulation vs text among COVID-19 vaccine-hesitant adults during the omicron wave in Germany. JAMA Netw. Open 6 , e2256208 (2023).
Wang, K. et al. A multi-country test of brief reappraisal interventions on emotions during the COVID-19 pandemic. Nat. Hum. Behav. 5 , 1089–1110 (2021).
Douglas, B. D., Ewell, P. J. & Brauer, M. Data quality in online human-subjects research: comparisons between MTurk, Prolific, CloudResearch, Qualtrics, and SONA. PLoS ONE 18 , e0279720 (2023).
Peer, E., Rothschild, D., Gordon, A., Evernden, Z. & Damer, E. Data quality of platforms and panels for online behavioral research. Behav. Res. Methods 54 , 1643–1662 (2022).
Kunda, Z. The case for motivated reasoning. Psychol. Bull. 108 , 480 (1990).
Hertwig, R. & Engel, C. Deliberate Ignorance: Choosing Not to Know , Vol. 29 (MIT Press, 2021).
Watson, D., Clark, L. A. & Tellegen, A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54 , 1063–1070 (1988).
Willemsen, M. C. & Johnson, E. J. Visiting the Decision Factory: Observing Cognition with MouselabWEB and Other Information Acquisition Methods , 21–42 (Psychology Press, 2011).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using stan. J. Stat. Softw. 80 , 1–28 (2017).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (R Foundation for Statistical Computing, 2021).
Bürkner, P.-C. & Vuorre, M. Ordinal regression models in psychology: a tutorial. Adv. Methods Pract. Psychol. Sci. 2 , 77–101 (2019).
Gabry, J., Simpson, D., Vehtari, A., Betancourt, M. & Gelman, A. Visualization in Bayesian workflow. J. R. Stat. Soc. A Stat. Soc. 182 , 389–402 (2019).
Carpenter, B. et al. Stan: a probabilistic programming language. J. Stat. Softw. 76 , 1–32 (2017).
Stan Development Team. RStan: The R Interface to Stan. R package version 2.21.7. https://mc-stan.org/ (2022).
Prelec, D. The probability weighting function. Econometrica 66 , 497–527 (1998).
Van Houtven, G., Johnson, F. R., Kilambi, V. & Hauber, A. B. Eliciting benefit–risk preferences and probability-weighted utility using choice-format conjoint analysis. Med. Decis. Mak. 31 , 469–480 (2011).
Download references
Acknowledgements
The funding for this project was provided by the Max Planck Society. We would like to thank Deborah Ain for editing the manuscript.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Center for Adaptive Rationality, Max Planck Institute for Human Development, Berlin, Germany
Kamil Fuławka, Ralph Hertwig & Thorsten Pachur
School of Management, Technical University of Munich, Munich, Germany
Thorsten Pachur
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: K.F., R.H., T.P. Methodology: K.F., T.P. Software, formal analysis, investigation, and visualization: K.F. Writing—original draft: K.F., R.H., T.P.
Corresponding author
Correspondence to Kamil Fuławka .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fuławka, K., Hertwig, R. & Pachur, T. COVID-19 vaccine refusal is driven by deliberate ignorance and cognitive distortions. npj Vaccines 9 , 167 (2024). https://doi.org/10.1038/s41541-024-00951-8
Download citation
Received : 11 December 2023
Accepted : 14 August 2024
Published : 14 September 2024
DOI : https://doi.org/10.1038/s41541-024-00951-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Moderna's updated COVID-19 vaccine for 2024-25 approved in Canada
Vaccine targets kp.2 variant, one of the latest offshoots of omicron.

Social Sharing
Health Canada authorized Moderna's updated COVID-19 vaccine on Tuesday to roll out in fall immunization campaigns.
The federal vaccine portal lists approval of Moderna's product.
Provinces and territories plan fall immunization campaigns aiming to protect people from severe hospitalizations and deaths from COVID-19.
Guidance from the National Advisory Committee on Immunization (NACI) strongly recommends updated COVID-19 vaccinations starting this fall for high-risk groups:
- All adults 65 and older.
- People living in long-term care and other group settings.
- People with underlying medical conditions that put them at higher risk of severe illness.
- Individuals in or from First Nations, Métis and Inuit communities.
- Members of racialized and other equity-deserving communities.
- Those who are pregnant.
- People who provide essential community services.
All other individuals aged six months and older may receive the vaccine, NACI recommended.
A spokesperson for Moderna said it will start delivering the product to the Public Health Agency of Canada in the next day or two and expects "a robust supply will be available in time for provincial and territorial vaccination campaigns, but specific timing depends on provinces."
Dr. Fahad Razak, an internist at St. Michael's Hospital in Toronto, said fresh vaccines are recommended, as the virus that causes COVID-19 continues to mutate or change in order to dodge being recognized by our immune system and to bind better for entering human cells.
Omicron offshoots circulating
Moderna said the vaccine targets the KP.2 variant, one of the latest offshoots of Omicron. Based on Canadian viral sequencing data, KP variants continue to dominate.
The Omicron variant of concern set off a massive wave of infections worldwide starting in November 2021.
Currently, the number of PCR tests coming back positive for the virus that causes COVID-19 at hospitals stands at 18 per cent nationally , compared with four per cent in April, when a comparable number of tests were performed, Razak said.
"What little wastewater that's [tested] now in the country is showing high levels of transmission, so we're in a wave," Razak said. "But we're not seeing a high number of people in hospital."
Why updated vaccines needed
Wastewater testing offers an early indicator of when respiratory viruses are on the rise, doctors and epidemiologists say.
"We clearly know now that the waves of these COVID infections, at least for the foreseeable future, will continue to occur," Razak said. "That will require rapid updated vaccines." He applauded Health Canada for shortening the window on approving the vaccine.

A spokesperson for Health Canada said on Tuesday the department anticipates issuing a decision about the updated Pfizer and Novavax COVID-19 vaccines over the next few weeks.
"For me, most important clinically is that we continue to have protection against severe disease," Razak said.
Since many people in high-risk groups did not get vaccinated in the spring, that can leave them susceptible over time to end up in hospital, he said.

Experts now say impacts of COVID-19 stretch well beyond initial illness
Reducing infections whenever possible also matters given the long-term consequences infections can have on health. Razak suggested vaccinations to prevent severe illness and improving indoor air quality, as well as taking steps not to expose others when you're sick to prevent infections in general.
In August, the federal government sent a notice to health professionals saying the previous COVID-19 vaccines targeting an earlier variant would no longer be available.
Health Canada said the decision was "part of regulatory and supply management best practices, consistent with the approach to annual influenza vaccines."
ABOUT THE AUTHOR

Amina Zafar covers medical sciences and health care for CBC. She contributes to CBC Health's Second Opinion, which won silver for best editorial newsletter at the 2024 Digital Publishing Awards. She holds an undergraduate degree in environmental science and a master's in journalism.
Related Stories
- Provinces told to pull existing COVID-19 vaccines ahead of arrival of updated shots
- COVID shots available in B.C. until new vaccines arrive: province
- No Novavax COVID-19 vaccine in Canada this fall, immunocompromised N.B. woman feels 'expendable'
- Canada approved multiple RSV shots to ward off infections this fall — here's how to get them
- Manitoba not covering RSV treatments for newborns, pregnant people now recommended by federal agency
Add some “good” to your morning and evening.
A vital dose of the week's news in health and medicine, from CBC Health. Delivered to your inbox every Saturday morning.
This site is protected by reCAPTCHA and the Google Privacy Policy and Google Terms of Service apply.

Health Canada approves updated Moderna COVID-19 vaccine

Doses of Moderna's updated COVID-19 vaccine are expected to begin arriving in Canada "within days," a spokesperson for the Public Health Agency of Canada says, although availability will depend on the immunization rollout in each province and territory.
Health Canada announced Tuesday it had authorized Moderna's latest COVID-19 vaccine that protects against currently circulating variants of the virus.
- The information you need to know, sent directly to you: Download the CTV News App
The mRNA vaccine, called Spikevax, has been reformulated to target the KP.2 subvariant of Omicron, it said.
The updated version replaces the previous formulation of the vaccine that was released last year, which targeted the XBB.1.5 subvariant of Omicron.
The timelines for when people can roll up their sleeves to get the new shot is up to the provinces and territories, Public Health Agency of Canada spokesperson Anna Maddison said in an email Tuesday.
"Canada has secured sufficient supply of COVID-19 vaccines to meet provincial and territorial demand requirements for fall and winter 2024 vaccination campaigns," she said.
Health Canada recently asked provinces and territories to get rid of their older COVID-19 vaccines to ensure the most current vaccine will be used during this fall's respiratory virus season.
It is also reviewing two other updated COVID-19 vaccines but has not yet authorized them. They are Pfizer's Comirnaty, which is also an mRNA vaccine, as well as Novavax's protein-based vaccine.
"Health Canada anticipates issuing a decision regarding the Novavax and Pfizer COVID-19 vaccines over the next weeks," Maddison said.
Like Moderna's vaccine, the Pfizer vaccine under review targets the KP.2 strain. The Novavax vaccine targets the JN.1 variant. KP.2 is a sublineage of JN.1.
The JN.1 group, including its sublineages, continues to be the dominant lineage group in Canada, according to the Public Health Agency of Canada's COVID-19 update page.
Moderna's vaccine is approved for adults and children six months of age and older, the company said in a news release Tuesday.
"With vaccines ready, Moderna will begin delivery of updated doses to the Public Health Agency of Canada, ensuring supply is available in time for provincial and territorial vaccination campaigns," the release said.
In May, the National Advisory Committee on Immunization issued guidance for use of updated COVID-19 vaccines this fall, pending their approval by Health Canada.
In that guidance, NACI strongly recommended updated COVID-19 vaccinations for all adults 65 and older, people living in long-term care and other group living settings, people with underlying conditions that put them at higher risk of severe illness, people from Indigenous and racialized communities, and those who are pregnant or who provide essential community services.
- Top health headlines, all in one place
NACI also said all other adults and children six months or older should also be eligible for an updated COVID-19 vaccination this fall.
As of Sept. 8, the viral activity level of COVID-19 in this country is "moderate," according to Public Health Agency of Canada's wastewater testing data.
Levels of other respiratory diseases -- specifically influenza and RSV -- in wastewater are currently "low."
This report by The Canadian Press was first published Sept. 17, 2024.
Canadian Press health coverage receives support through a partnership with the Canadian Medical Association. CP is solely responsible for this content.
CTVNews.ca Top Stories

Affordability crisis could be reaching its peak in Canada, economist says
With Canada's annual inflation rate reaching the central bank's two per cent target, the country's affordability crisis could be peaking, according to an economist.
Record-breaking Lotto Max jackpot tickets sold in Ontario, Quebec
Two lucky people in Ontario and Quebec will split Tuesday’s record-breaking $80-million Lotto Max jackpot.
Rogers Communications to buy out Bell's share of MLSE for $4.7 billion
Rogers Communications Inc. is buying out Bell's 37.5 per cent share of Maple Leaf Sports & Entertainment for $4.7 billion, giving it 75 per cent ownership of the sports conglomerate.
Catherine, Princess of Wales, goes back to work days after cancer treatment update
Catherine, Princess of Wales has held her first engagement since revealing that she has completed her chemotherapy treatment.
8-year-old Ohio girl takes her family's SUV, drives to Target
An 8-year-old girl took an SUV from her Ohio home and drove for miles to a store where she was later found unharmed, authorities said.
Bride's family speaks as West Vancouver woman sentenced for driving SUV into wedding party
Sixty-five-year-old Hong Xu, who drove her SUV into a crowd of people celebrating a wedding at her next-door neighbour's house in West Vancouver on Aug. 20, 2022, has been sentenced under the Motor Vehicle Act for driving without due care and attention.
Ukrainian drones strike a large military depot in a Russian town northwest of Moscow
Ukrainian drones struck a large military depot in a town deep inside Russia overnight, causing a huge blaze and prompting the evacuation of some local residents, a Ukrainian official and Russian news reports said Wednesday.
How to prevent lung cancer, regardless of whether you smoke, according to a doctor
More people who have never touched a cigarette are getting lung cancer, but there are ways to prevent it, according to a doctor.
This airport landing is so challenging only 50 pilots are qualified to do it
Bhutan's Paro International Airport (PBH) is widely considered one of the most technically difficult plane landings in the world. Maneuvering onto a short runway between two 18,000-foot peaks requires both technical knowledge and nerves of steel.

Man from Phoenix, Ariz., missing after truck plunges off Yukon bridge
Whitehorse RCMP say a man from Phoenix, Ariz., is missing after the truck he was travelling in went off a bridge and plunged into the Yukon River.
'It's disappointing': Kinew responds to ousted MLA's claims of toxic, dysfunctional government
Manitoba Premier Wab Kinew said an MLA ousted from his caucus this week was given a choice before he was shown the door.
Two people charged in murder of Halifax teen; police believe remains have been found
Halifax Regional Police believe Devon Sinclair Marsman, who disappeared in 2022, was the victim of a homicide and two people have now been charged in his death.
Former B.C. teacher sent messages with 'sexual references' to Grade 7 girls: regulator
A former B.C. teacher committed misconduct when he sent inappropriate messages – including some with 'sexual references and innuendo' – to girls while he was their Grade 7 teacher, according to the professional regulator.

U.S. court upholds British socialite Ghislaine Maxwell's sex trafficking conviction
A U.S. court on Tuesday upheld disgraced British socialite Ghislaine Maxwell's conviction on sex trafficking charges for helping the late financier Jeffrey Epstein abuse underage girls.

Pakistan police arrest key suspect in gang rape of a woman polio worker
Pakistani police arrested the key suspect in the gang rape of a woman polio worker who was assaulted by three men during last week's vaccination campaign, officials said Wednesday. Two other suspects are still at large.
Cabin pressure issue on Delta flight causes bloody ears and noses for passengers
Some Delta Air Lines passengers are recovering after a pressurization issue on a flight caused bloody noses and other issues, according to statements.
Exploding Hezbollah pagers in apparent Israeli attack made by Hungarian company, Taiwanese firm says
A company based in Hungary was responsible for manufacturing the pagers that exploded in Lebanon and Syria in an apparent Israeli operation targeting Hezbollah’s communications network, another firm whose brand was used on the devices said Wednesday.
Rising rivers threaten southern Poland as flooding recedes elsewhere in Central Europe
Soldiers and volunteers in southwestern Poland were laying sandbags Wednesday near swollen rivers in the region of Wroclaw as they worked to safeguard homes and businesses after days of flooding across Central Europe.

Poilievre's first chance to topple Trudeau government expected next week
Conservative Leader Pierre Poilievre is set to get his first chance to topple Prime Minister Justin Trudeau's minority Liberal government next week, CTV News has confirmed.
'Say it to my face': Singh confronts heckling protester on Parliament Hill
NDP Leader Jagmeet Singh confronted a protester for calling him a 'corrupted bastard' on Parliament Hill on Tuesday.
Public inquiry to hear from current, former MPs targeted by foreign meddling
A federal inquiry into foreign interference is slated to hear today from current and former politicians who have been singled out by meddlers.

Doses of Moderna's updated COVID-19 vaccine are expected to begin arriving in Canada 'within days,' a spokesperson for the Public Health Agency of Canada says, although availability will depend on the immunization rollout in each province and territory.
These people say they got listeria after drinking recalled plant-based milks
The Canadian Press spoke to 10 people, from the parents of a toddler to an 89-year-old senior, who say they became sick with listeria after drinking from cartons of plant-based milk stamped with the recalled product code. Here's a look at some of their experiences.

'Ghost' cybercrime platform dismantled in global operation, 51 arrested
An international law enforcement operation has dismantled an encrypted communication platform, known as Ghost, notorious for enabling large-scale drug trafficking and money laundering, Europol said on Wednesday.
Qualcomm loses court appeal against European Union antitrust penalty in chipset case from 2019
Qualcomm lost its bid on Wednesday to get a European Union antitrust penalty thrown out after a top court largely rebuffed the technology company's arguments in the case involving cellphone chipsets.
23andMe settles data breach lawsuit for US$30 million
23andMe will pay US$30 million and provide three years of security monitoring to settle a lawsuit accusing the genetics testing company of failing to protect the privacy of 6.9 million customers whose personal information was exposed in a data breach last year.
Entertainment

Taylor Swift previously said she was uninspired to include politics in her music. Now, she's singing a different tune
In 2011, a young Taylor Swift said she was not inspired to sing about topics related to politics. Over a decade later, she's singing a different tune.
JD Souther, a singer-songwriter who penned hits for the Eagles and Linda Ronstadt, dies at 78
John David 'JD' Souther, a prolific songwriter and musician who helped shape the country-rock sound that took root in Southern California in the 1970s with his collaborations with the Eagles and Linda Ronstadt, has died at the age of 78.
'Fake heiress' Anna Sorokin debuts on 'Dancing with the Stars', with a sparkly ankle monitor
Convicted con artist Anna Sorokin has hit the dancefloor on 'Dancing With the Stars' with a featherweight — and very sparkly — ankle monitor.
Inflation data reveals what cost more in Canada lately
Canadians are still feeling the pinch when it comes to shopping for certain items and living expenses, even as inflation has cooled, according to Statistics Canada's new data released Tuesday.
U.S. Federal Reserve is set to cut interest rates for the first time in 4 years
Having all but tamed inflation, the U.S. Federal Reserve is poised to cut its benchmark interest rate, a step that should lead to lower borrowing costs for consumers and businesses just weeks before the presidential election.

Trip down memory lane: Seniors watch 'Grease' at B.C. care home's simulated drive-in
Summer days are drifting away, but a group of B.C. seniors had one lively summer night this week – watching "Grease" at a simulated drive-in movie theatre.
Manitoba newlyweds win $3 million in lottery
Newlyweds from Starbuck, Man. are starting their future together with a multi-million-dollar nest egg thanks to a big lottery win.

A'ja Wilson and rookie Caitlin Clark smash WNBA records
Las Vegas Aces superstar A’ja Wilson and Indiana Fever rookie Caitlin Clark both broke WNBA records Sunday, with Wilson becoming the first player to score 1,000 points in a single season and Clark breaking the rookie scoring record.
Canucks' Dakota Joshua reveals he is recovering from cancer
Vancouver Canucks forward Dakota Joshua revealed Tuesday he underwent cancer treatment over the summer, and will not be ready to play when the team's training camp begins later this week.
Toronto Maple Leafs unveil new logo on helmets
The Maple Leafs unveiled their new helmet partner that really puts the 'o' in Toronto.

Some Ontario EV plants are hitting the brakes. Does that mean Canada's ambitions are under threat?
The plant was expected to produce batteries for a million electric vehicles a year. Once up and running, it was supposed to create hundreds of permanent jobs in a small southeastern Ontario municipality. But two years later, spending on the construction of the Umicore plant has been delayed in what the company calls a "significant worsening of the EV market context."
Classic car in the family since 1958 stolen from Winnipeg garage
A Winnipeg man is asking for help after a classic car that has been a part of his family since the 1950s was stolen from his garage.
Ontario driver caught going more than 100 km/h in school zone
A 22-year-old driver was caught going more than 100 km/h in a school zone in York Region on Monday.
Local Spotlight

'The gift they gave us was their service': 50 years since first female troop joined the RCMP
The Royal Canadian Mounted Police is celebrating an important milestone in the organization's history: 50 years since the first women joined the force.
Young family from northern Ontario wins $70 million Lotto Max jackpot
It's been a whirlwind of joyful events for a northern Ontario couple who just welcomed a baby into their family and won the $70 million Lotto Max jackpot last month.
'The right thing to do': Good Samaritan builds new bottle cart for Moncton man who had his stolen
A Good Samaritan in New Brunswick has replaced a man's stolen bottle cart so he can continue to collect cans and bottles in his Moncton neighbourhood.
Oppenheimer star David Krumholtz dishes on his time filming in Winnipeg
David Krumholtz, known for roles like Bernard the Elf in The Santa Clause and physicist Isidor Rabi in Oppenheimer, has spent the latter part of his summer filming horror flick Altar in Winnipeg. He says Winnipeg is the most movie-savvy town he's ever been in.
'Craziest thing I've ever seen': Elusive salamanders make surprising mass appearance in Edmonton area
Edmontonians can count themselves lucky to ever see one tiger salamander, let alone the thousands one local woman says recently descended on her childhood home.
'A nightmare': Nature-goers stranded in B.C. backcountry after bridge washes out
A daytrip to the backcountry turned into a frightening experience for a Vancouver couple this weekend.

B.C. woman reveals greatest life lesson after celebrating 100th birthday
If you take a look to the right of Hilda Duddridge’s 100th birthday cake, you’ll see a sculpture of a smiling girl extending her arms forward.
Sisters finally see the Canadian 'aviation artifact' built by their father nearly 90 years ago
Two sisters have finally been reunited with a plane their father built 90 years ago, that is also considered an important part of Canadian aviation history.
The debate over taking horns off Viking statue in Gimli
A Facebook post has sparked a debate in Gimli about whether to make a cosmetic change to its iconic statue.
NDP defends Surrey Memorial Hospital efforts after damning letter from doctors
The BC NDP government is on the defensive Tuesday after emergency room doctors at Surrey Memorial Hospital penned a damning call for a leadership change at Fraser Health.
Downtown Vancouver BIA launches public safety campaign ahead of B.C. election
The business association for the downtown core is calling on parties running candidates in the upcoming provincial election to lay out their visions for improving public safety in Vancouver.

Michelin to award new stars in Toronto area as it expands outside city limits
The Michelin Guide's famous inspectors have ventured outside Toronto's city limits.

Calgary city council votes to wind down long-sought, long-troubled Green Line LRT project
Calgary city council has voted to end work on the first phase of its long-sought $6.2-billion Green Line light rail transit project at a cost of at least $2.1 billion.
Alberta to boost spending on new K-12 school construction over next three years by $6.5B to $8.6B
Alberta's premier announced a plan Tuesday evening during a televised address her government will boost the amount of money being spent on new school construction over the next three years to $8.6 billion, an increase of $6.5 billion from what was originally promised in the 2024 budget.
Rent, mortgage costs still concerning for Albertans despite cooling inflation
Canada’s annual inflation rate has reached the central bank’s two per cent target for August, but many consumers in Alberta are still feeling the pinch of high costs for shelter, rents and mortgages.

You will be able to 'dine-in-the-dark' at this new Ottawa restaurant
Dark Fork has announced plans to open the city's first "dine-in-the-dark restaurant" on George Street, where patrons will eat in a dark dining room where cellphones and other sources of artificial light are forbidden.
Woman missing, man rescued on Ottawa River after going out in a kayak, canoe
A search is underway in the Ottawa River for a 30-year-old woman reported missing while kayaking near Pembroke, Ont.
Ottawa City Council to debate restoring off-peak LRT service
Ottawa City Council meets Wednesday and a proposed motion to restore off-peak LRT service is on the agenda.

Black Lives Ruined: Black men asked to sign NDAs to settle racial profiling cases
Black men who are the victims of racial profiling and harassment by police forces in Quebec say they are being asked to sign non-disclosure agreements in order to receive their settlement cheques.
Quebecer wins big in historic $80M Lotto Max jackpot
A Quebecer is one of two people who won big in the record-breaking $80 million Lotto Max jackpot.
16 soldiers injured in accident at Valcartier military base in Quebec
More than a dozen soldiers were sent to hospital this afternoon after an accident at a military base in Quebec.

BREAKING | Several homes on fire in southwest Edmonton
Flames spread from an apartment building under construction to several townhomes in southwest Edmonton early Wednesday morning.
'A matter of luck whether or not you make it out alive': Excessive speeders taunt police by posting crimes online
Whether you've been shaken awake by revving engines in the dead of night, or passed on the Anthony Henday like you're standing still, most Edmontonians have some experience with sports cars or motorcycles driving dangerously on city streets.

Expert says warm September in Maritimes could cause higher aggression in bees and wasps
Experts say the higher Maritime temperatures make wasps and hornets more territorial and aggressive in the late summer.
Check your ticket: Lotto Max ticket worth $1M sold in New Brunswick
Someone in New Brunswick has a million reasons to smile, according to Atlantic Lottery.
Why it's 'very hard' to find work in Canada
Vacancies have steadily fallen since the glut of nearly one million open posts in 2022. At the time, one in three businesses had trouble hiring staff due to a labour shortage. Since then, vacancies have dropped.

How much rain fell in southern Manitoba over the past few days
Southern Manitoba was hit with torrential downpours, overland flooding and thunderstorms at the start of the week, with some communities receiving upwards of 200 millimetres (mm) of rain.
Prosthetic leg, live goldfish among items left on Winnipeg buses
There's nothing average about some of the stuff that gets lost on the bus

Suspect still at large following assault investigation in Yorkton: RCMP
Three suspects have been arrested, while one remains at large, following an assault in the city of Yorkton.
Tornado warning for parts of southwestern Sask. lifted
A tornado warning, which was issued for parts of southwestern Saskatchewan on Tuesday evening, has been lifted.
'Didn't meet our expectations': Tempers flare at Riders practice with team winless in 7
It was a heated day at practice for the Saskatchewan Roughriders on Tuesday as head coach Corey Mace had to address the team in a stern matter not once but twice resulting in him telling the group to, “Get off the f***ing field.”
City of Guelph proposes bylaw to protect renters
City council approved an 'evictions survey' during Tuesday night’s meeting to get a better snapshot of the impacts of evictions occurring within Guelph.
Cyclist dies following collision with pickup truck
Wellington County Ontario Provincial Police responded to a serious motor vehicle collision on Wellington Road 18, just west of Salem.

Emily Sanche tried to seek medical help for boyfriend before he fatally stabbed her, court hears
Catherine Sanche says her cousin and best friend Emily Sanche never feared her boyfriend Thomas Hamp would hurt her in the weeks leading up to her death in February 2022.
Support staff at Saskatoon public schools call for more safety supports after teen set on fire
Support staff at Saskatoon Public Schools are calling for urgent action and more funding to keep members safe in the wake of a brutal attack at Evan Hardy Collegiate earlier this month.
Another person charged at Sask. private school at the centre of multiple abuse allegations
Another person affiliated with a Saskatoon Christian school embroiled in legal trouble over multiple allegations of abuse has been charged with assault.
Northern Ontario

One person dead following North Bay industrial incident
North Bay Police Service says one person has died following an industrial accident at the Ontario Northland Transportation Commission Rail Yard on Tuesday.
'It's ridiculous': Ontario man told to pay $1,000 to end water heater contract
An Ontario man was surprised to learn he would have to pay a $1,000 penalty to cancel his water heater rental. 'I was shocked that the penalty I had to pay was almost the cost of a brand new water heater,' James Alves, of Etobicoke, told CTV News Toronto.
GoFundMe cancels fundraiser for Ontario woman charged with spraying neighbour with a water gun
A Simcoe, Ont., woman charged with assault with a weapon after accidentally spraying her neighbour with a water gun says GoFundMe has now pulled the plug on her online fundraiser.
Perth County crash sends motorcycle driver to hospital
A motorcycle driver has serious injuries after a crash in Perth County. It happened around 8 p.m. int he area of Highway 8 between Perth Road 179 and Perth Road 168.
Pets stolen in break and enter returned to owner
South Bruce OPP were able to reunite an owner and their pets after they were taken from their home.

Weekend GO Transit Barrie Line closed for repairs
GO Transit will be using buses on the Barrie Line this weekend due to track upgrades and repairs.
People posing as Friends are allegedly scamming Friends on Facebook: OPP
Ontario Provincial Police are warning social media users about recent scams.
New clinic for kids providing hospital alternative
As hospital emergency departments continue to be overwhelmed amidst an ongoing shortage of physicians in Ontario, there's a new Orillia operation hoping to ease the pressure.

Pedestrian in ICU after getting struck by vehicle
Windsor police say a pedestrian was taken to hospital with serious injuries after getting hit by a vehicle in south Windsor.
Ward 6 kicks off series of meetings in the city
Windsor ward meetings have begun, with councillor conversations and resident reservations. Coun. Ed Sleiman was up first in the series of meetings, giving residents a public forum for face time with councillors and city officials.
Vancouver Island

'Certainly a wake-up call': B.C. police remind residents to lock their doors after family robbed
Mounties on Vancouver Island are warning people to lock their doors after a Nanaimo-area family was robbed of a high-end vehicle, cash, a computer and other merchandise while they slept.
B.C. forest watchdog says province should improve watershed management
British Columbia's forests watchdog says a complaint about "excessive" logging has led to a call for the province to improve how it manages watersheds.

Pregnant pit bull with 10 puppies rescued from rat-infested B.C. home
Animal protection officers in British Columbia have rescued three pit bulls – including one that gave birth to 10 puppies – from a rat-infested home in Kelowna.
Son charged with B.C. woman's murder: RCMP
More than a year after a missing Kamloops, B.C., woman’s body was found, her son has been arrested and charged with her murder, Mounties announced Friday.
Woman stabbed during daylight Kelowna home invasion: RCMP
A woman suffered life-threatening injuries after being stabbed during a home invasion in Kelowna, according to authorities.

Lethbridge sees spike in encampments, and in support referrals
The City of Lethbridge's encampment response team continues to see a rise in the number of people experiencing homelessness throughout the summer months.
2 Lethbridge youths arrested after fire at southeast business
Two 16-year-old boys have been charged with arson following a fire at a Lethbridge building earlier this month.
Lethbridge residents used 16 per cent less water this summer than last
The City of Lethbridge says water usage this summer was lower than expected.
Sault Ste. Marie
Car trouble in northern ontario results in drug bust.
Three northern Ontario residents are charged with drug trafficking after the vehicle they were in got stuck along a bush road off Highway 17 on Monday.
Sault man charged with threating victim with a hammer
A 50-year-old man in the Sault has been charged following an assault on Saturday that involved the use of a hammer.

Newfoundland and Labrador monitoring rise in whooping cough cases: medical officer
Newfoundland and Labrador's chief medical officer is monitoring the rise of whooping cough infections across the province as cases of the highly contagious disease continue to grow across Canada.
Dispute over unrecognized Inuit group halts major conference for Canadian North
A 16-year-old biennial event aimed at fostering business in the country's eastern Arctic and northern regions has been cancelled indefinitely as a dispute unfolds between Inuit in Canada and a Labrador group claiming to share their heritage.
Cow cuddling: Why a Newfoundland farm is offering quality time with these 'gentle creatures'
Jim Lester’s farm hopped on the cow-cuddling trend in early August, and his time slots have been pretty well sold out ever since.
Shopping Trends
The Shopping Trends team is independent of the journalists at CTV News. We may earn a commission when you use our links to shop. Read about us.
Editor's Picks
These silk & snow sheets stopped me and my partner from arguing over bedding (and they're on sale right now), 14 products that'll help you live your best, coziest life this fall, school has officially begun, and here are a few essential supplies that won't break the bank, 14 pieces of fall decor that’ll give your home autumnal vibes, these smart cleaning products will make doing your chores way easier, our guide to the best smart thermostats in canada in 2024 (and where to get them), 15 of the best birthday gifts to give this month, 18 top-notch presents and gift add-ons that anyone would love to receive, 15 of the best gifts to give a one-year-old for their first birthday, the best gels, pomades, and waxes that'll keep your hair in place, these beauty and skincare products will help you create a flawless base, these 12 setting sprays will actually keep your makeup from budging, from wayfair to walmart: here are the best deals you'll find online this weekend, from anthropologie to amazon: here are the best deals you'll find online this weekend, beauty week has officially begun on amazon canada — here are the best deals you can find, stay connected.

- Search Search Please fill out this field.
- Markets News
- Stocks & Bond News
Moderna Stock Jumps on Canadian Government's Approval of Updated COVID-19 Vaccine
Bill McColl has 25+ years of experience as a senior producer and writer for TV, radio, and digital media leading teams of anchors, reporters, and editors in creating news broadcasts, covering some of the most notable news stories of the time.
:max_bytes(150000):strip_icc():format(webp)/McCollresize-752ea47dccf24c979ced3f694a0e7361.jpg)
UCG / Contributor / Getty Images
Key Takeaways
- Moderna shares surged Tuesday after Canadian regulators approved the biotech firm’s updated COVID-19 vaccine.
- Moderna's Spikevax KP.2 targets the KP.2 variant of the virus.
- The U.S. Food and Drug Administration has also approved the shot, as well as updated vaccines from Pfizer and BioNTech.
Moderna ( MRNA ) shares surged Tuesday after Canadian regulators approved the biotech firm’s updated COVID-19 vaccine.
The company reported Health Canada authorized the use of its Spikevax KP.2 shot for those aged six months and older. Last month, the U.S. Food and Drug Administration also gave approval for the vaccine, as well as updated shots from Pfizer ( PFE ) and BioNTech ( BNTX ).
Moderna's Spikevax KP.2 targets the KP.2 variant of the virus, which is a descendant of the JN.1 variant that began appearing last winter.
Moderna said now that it has received approval, the company will begin delivering the Spikevax KP.2 to the Public Health Agency of Canada.
Shares of Moderna were up nearly 4% at $71.81 in afternoon trading Tuesday following the news. Despite Tuesday's gains, they've lost more than one-quarter of their value since the start of the year.
TradingView
Moderna. " Moderna Receives Health Canada Approval For Updated COVID-19 Vaccine Targeting KP.2 Variant Of SARS-COV-2 For Ages Six Months And Older ."
Food and Drug Administration. " FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants ."
:max_bytes(150000):strip_icc():format(webp)/GettyImages-2136074452-500b982af2ed4ed091a6380b7e117cb5.jpg)
- Terms of Service
- Editorial Policy
- Privacy Policy
We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here . By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service .

New to Zacks? Get started here.
Member Sign In
Don't Know Your Password?

- Zacks #1 Rank
- Zacks Industry Rank
- Zacks Sector Rank
- Equity Research
- Mutual Funds
- Mutual Fund Screener
- ETF Screener
- Earnings Calendar
- Earnings Releases
- Earnings ESP
- Earnings ESP Filter
- Stock Screener
- Premium Screens
- Basic Screens
- Thematic Screens
- Research Wizard
- Personal Finance
- Money Management
- Retirement Planning
- Tax Information
- My Portfolio
- Create Portfolio
- Style Scores
- Testimonials
- Zacks.com Tutorial
Services Overview
- Zacks Ultimate
- Zacks Investor Collection
- Zacks Premium
Investor Services
- ETF Investor
- Home Run Investor
- Income Investor
- Stocks Under $10
- Value Investor
- Top 10 Stocks
Other Services
- Method for Trading
- Zacks Confidential
Trading Services
- Black Box Trader
- Counterstrike
- Headline Trader
- Insider Trader
- Large-Cap Trader
- Options Trader
- Short Sell List
- Surprise Trader
- Alternative Energy

You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK . If you do not, click Cancel.
Image: Shutterstock
Why Novavax is Gaining Ground in COVID-19 Vaccine Race
Covid-19 positive rate spikes.
With more knowledge about the virus, ample funding, and new treatments, the worst of the COVID-19 pandemic seems to be behind us (from a death perspective). However, just because the Coronavirus death rate has dropped dramatically since its major peak in 2020 (and a subsequent spike in 2021) doesn’t mean that COVID-19 is still not negatively impacting people who get it.
Data from the CDC website shows that the number of people testing positive for COVID-19 has reached its highest level since early about two years.

Though fewer people are dying from the virus, “long COVID” is something that people want to avoid because it can cause complications (not yet fully understood) that can include extreme fatigue, digestion issues, and potential brain issues in younger patients.
Novavax: A New Entrant in the COVID-19 Vaccine Realm
From a vaccine perspective, the two biggest winners from the COVID-19 vaccine race were Pfizer ( ( PFE Quick Quote PFE - Free Report ) ) and Moderna ( ( MRNA Quick Quote MRNA - Free Report ) ). However, with COVID-19 stubbornly sticking around, investors should focus on an obscure, up-and-coming COVID-19 vaccine maker called Novavax.
Novavax Sanofi Deal Offers Distribution and Funding
Novavax ( ( NVAX Quick Quote NVAX - Free Report ) ) is a biotech company that develops innovative vaccines to prevent serious infectious diseases. In May, the company entered into a multi-billion-dollar partnership with French biotech giant Sanofi ( ( SNY Quick Quote SNY - Free Report ) ). NVAX shares jumped 80% for the week when the deal was announced because it offered NVAX a critical component it did not have – distribution.
Per the deal terms, Sanofi took a minority stake in NVAX (a $70 million equity investment), will gain rights to co-market Novavax’s COVID-19 vaccine globally, and will have the sole license to develop and market NVAX’s COVID vaccine in combination with its influenza vaccine. NVAX also received a payment of $570 million from Sanofi, which was included in the deal.
Florida DOH Advises Against mRNA COVID-19 Vaccine
Distribution is one way that Novavax can tighten the vaccine race and catch up to more prominent players like PFE and MRNA. Another way is through its differentiated, non-mRNA vaccine. Friday, Moderna shares dove more than 12% on massive volume after the Florida Department of Health (DOH) advised against mRNA COVID-19 vaccines, citing seven safety and efficacy concerns. Novavax, which uses protein-based vaccines, is the clear beneficiary. NVAX shares jumped 14% Friday in reaction to the news.

COVID-Influenza Combo Vaccine Could be Game-Changer
Novavax’s COVID-influenza drug is slated to reach late-stage trials by the end of 2024. Should the drug pass trials, this unique, first-of-its-kind drug should act as a bullish catalyst into year-end.
NVAX Stock's Bullish Chart Pattern
NVAX’s share price and volume action is mimicking its strong fundamental possibilities. The stock is carving out a bullish monthly bull flag pattern. Shares should accelerate to the upside if they can clear last month’s hammer candle highs of $14.09.

Bottom Line
Though Novavax is behind in the COVID-19 vaccine race, a blockbuster deal with Sanofi and a differentiated product means the stock offers the best reward prospects in the industry moving forward.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report free:.
Sanofi (SNY) - free report >>
Pfizer Inc. (PFE) - free report >>
Moderna, Inc. (MRNA) - free report >>
Novavax, Inc. (NVAX) - free report >>
Published in
This file is used for Yahoo remarketing pixel add
Due to inactivity, you will be signed out in approximately:
New York Immigrant Health Care News
You Need An Updated COVID-19 Vaccine: Get One For Free in NYC
This guide will walk you through how to get the updated covid-19 vaccine for free in nyc, even if you are undocumented or uninsured.
Sep 18, 2024
Here is how to get a vaccine to protect against COVID-19 in NYC.
This guide will walk you through how to get the updated COVID-19 vaccine for free in NYC, even if you are undocumented or uninsured.
Why should I get the updated COVID-19 shot?
COVID-19 is constantly evolving, and the protection from older vaccines fades over time. The updated 2024-2025 vaccine is designed to protect against the newest variants that are causing infections and hospitalizations in the U.S. By getting the updated shot, you reduce your chances of getting seriously ill or hospitalized due to COVID-19. It also helps lower the risk of developing Long COVID , a condition where symptoms can last for months after the initial infection.

Last year, those who received the 2023-2024 COVID-19 vaccine had better protection from serious illness than those who did not.
Am I eligible to get the updated COVID-19 vaccine?
Yes! Everyone aged 6 months and older is eligible to get the updated COVID-19 vaccine, no matter your residency, immigration status, or insurance coverage. You do not need U.S. citizenship or health insurance to receive the vaccine.
Where can I get an updated COVID-19 shot in NYC?
If you are uninsured or undocumented, here are some places in NYC where you can get the vaccine for free:
NYC Health + Hospitals Facilities NYC’s public hospitals and clinics offer free COVID-19 vaccines to everyone, including those who are undocumented or uninsured. These locations are safe and welcoming to all. Visit the NYC Health + Hospitals website to find a location near you: NYC Health + Hospitals
HRSA-Funded Community Health Centers These centers provide medical care on a sliding scale based on your income and family size. If you can’t afford to pay, they will still give you the vaccine for free. Undocumented individuals are welcome, and no one will be turned away. Find a health center near you through the following link: Find a Health Center
NYC Vaccine Finder Use the NYC Vaccine Finder website to locate a nearby vaccination site. You can filter the search to find locations offering free or low-cost vaccines, specifically for uninsured individuals. Many of these sites do not require any form of insurance. Visit NYC Vaccine Finder .
Please note that COVID-19 vaccines are no longer available for free to uninsured individuals at major pharmacies such as CVS, following the end of the federally funded program.
“As you know, the CDC’s Bridge Access Program, which provided no-cost COVID-19 vaccines to uninsured and underinsured adults, has ended. While CVS is not offering free COVID-19 vaccines, patients could check with their local health department to see if any free or low-cost clinics are being offered,” said Amy Thibault, Lead Director of External Communications at CVS Pharmacy.
When will the updated COVID-19 shot be available at these locations?
The 2024-2025 COVID-19 vaccines are now available following the FDA’s recent approval of Pfizer, Moderna, and Novavax vaccines. Vaccines are being shipped directly from manufacturers to healthcare providers and pharmacies, with many sites expected to begin vaccinations by early to mid-September.
The NYC Vaccine Finder will be updated with locations where you can get the new vaccine when it is available. You can also contact your healthcare provider to check when they will have the vaccine ready.
Is there language support available for non-English speakers at these vaccination sites?
City-managed sites, such as NYC Health + Hospitals clinics and the Health Department’s Fort Greene Health Center immunization clinic , offer language line translation services .

Immigration News Today: New NYC Data on Migrants and Shelters
For additional resources on the updated COVID-19 vaccines, you can visit the NYC Health Department’s COVID-19 Vaccine page , which provides multilingual information.
What documents do I need to get a COVID-19 vaccine?
An ID is not required for a vaccination.
Everyone who lives in the United States is eligible to receive free COVID-19 services, including vaccines, testing, and treatment, even if you do not have insurance, and no matter your immigration status. You do not need a Social Security Number or government ID to receive free COVID-19 services.
Additional information for undocumented and uninsured people
You do not need to share your immigration status when getting the COVID-19 vaccine. Vaccination sites will not report you to immigration authorities, and your information will remain confidential. NYC is committed to ensuring that everyone, regardless of status, has access to health care, including the COVID-19 vaccine.
Faye Qiu, Documented's Chinese Community Correspondent, is deeply connected to New York City’s Chinese community, with a career dedicated to supporting underserved immigrant populations. Currently, Faye serves as the Community Outreach Coordinator for the Committee of 100, where she leads efforts for the AAPI Initiative to combat the underreporting of anti-Asian hate incidents. She remains committed to serving her community through weekly volunteer work with the Chinese Consolidated Benevolent Association in Chinatown.
TOP STORIES

Public Safety
Law Enforcement Can’t Confirm the Tren de Aragua Gang is in New York City
BY Maurizio Guerrero

Arts & Culture New York Immigrant Communities
Citywide Art Campaign Reconnects New York with Its Immigrant Roots
BY Rommel H. Ojeda
SEE MORE STORIES

Most Latinx Immigrants Didn’t Know Abortion Is Legal in New Jersey. Their Community Is Spreading the Word.

Making Mooncakes: Celebrating The Mid-Autumn Festival in Brooklyn
Early Arrival Newsletter
Receive a roundup of immigration and policy news from New York, Washington, and nationwide in your inbox 3x per week.

Queens Street Vendors Fight Back as NYPD Crushes Food Carts
Uber still locks drivers out of the app despite nyc deal.

Kamala Harris and Immigration: Her Record With Asylum Seekers
What happens to migrants and asylum seekers if trump becomes president.

Doula Birth and Postpartum Services for Black Women in NYC
Back-to-school fair helps immigrant families gear up for new school year.

Immigration Experts Debrief the Presidential Debate
At presidential debate, trump delivers lies on immigration. harris attacks with policy proposal.

PO Box 924 New York, NY 10272
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Transl Sci
- v.14(6); 2021 Nov
COVID‐19 vaccine hesitancy: Race/ethnicity, trust, and fear
Don e. willis.
1 College of Medicine, University of Arkansas for Medical Sciences Northwest, Fayetteville Arkansas, USA
Jennifer A. Andersen
Keneshia bryant‐moore.
2 Fay W. Boozman College of Public Health, University of Arkansas for Medical Sciences, Little Rock Arkansas, USA
James P. Selig
Christopher r. long, holly c. felix, geoffrey m. curran.
3 College of Pharmacy, University of Arkansas for Medical Sciences, Little Rock Arkansas, USA
Pearl A. McElfish
Understanding and minimizing coronavirus disease 2019 (COVID‐19) vaccine hesitancy is critical to population health and minimizing health inequities, which continue to be brought into stark relief by the pandemic. We investigate questions regarding vaccine hesitancy in a sample ( n = 1205) of Arkansas adults surveyed online in July/August of 2020. We examine relationships among sociodemographics, COVID‐19 health literacy, fear of COVID‐19 infection, general trust in vaccines, and COVID‐19 vaccine hesitancy using bivariate analysis and a full information maximum likelihood (FIML) logistic regression model. One in five people (21,21.86%) reported hesitancy to take a COVID‐19 vaccine. Prevalence of COVID‐19 vaccine hesitancy was highest among Black/African Americans (50.00%), respondents with household income less than $25K (30.68%), some college (32.17%), little to no fear of infection from COVID‐19 (62.50%), and low trust in vaccines in general (55.84%). Odds of COVID‐19 vaccine hesitancy were 2.42 greater for Black/African American respondents compared to White respondents ( p < 0.001), 1.67 greater for respondents with some college/technical degree compared to respondents with a 4‐year degree ( p < 0.05), 5.48 greater for respondents with no fear of COVID‐19 infection compared to those who fear infection to a great extent ( p < 0.001), and 11.32 greater for respondents with low trust in vaccines ( p < 0.001). Sociodemographic differences in COVID‐19 vaccine hesitancy raise concerns about the potential of vaccine implementation to widen existing health disparities in COVID‐19 related infections, particularly among Black/African Americans. Fear of infection and general mistrust in vaccines are significantly associated with vaccine hesitancy.
Study Highlights
- WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Prior research has documented that coronavirus disease 2019 (COVID‐19) vaccine hesitancy varies greatly by race and ethnicity. However, many questions remain regarding patterns of vaccine hesitancy across sociodemographics and attitudes.
- WHAT QUESTION DID THIS STUDY ADDRESS?
What proportion of Arkansans are hesitant to get the COVID‐19 vaccine? How does COVID‐19 vaccine hesitancy differ across sociodemographic groups in Arkansas? How does knowledge of how to protect one’s self against the virus, fear of the virus, and general trust for vaccines relate to COVID‐19 vaccine hesitancy?
- WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study is the first to look at sociodemographic differences in COVID‐19 vaccine hesitancy in a highly vulnerable rural state that ranks third for prevalence of individuals at high risk for serious illness from COVID‐19. The COVID‐19 vaccine hesitancy was highest among respondents with lower household income, some college, and little to no fear of infection from COVID‐19.
- HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study can inform public health interventions aiming to reduce the unequal burden of COVID‐19 morbidity and mortality through equitable vaccine distribution and health communication.
INTRODUCTION
In January 2020, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—the etiological agent of coronavirus disease 2019 (COVID‐19)—was detected for the first time in a patient in the United States (US). By December 2020, COVID‐19 was the leading cause of death in the US, 1 with a disproportionate loss of life occurring among communities of color. 2 In December 2020, distribution of 2 COVID‐19 vaccines began across the US. 3 The next major goal is to achieve population immunity.
Population immunity is achieved when a large enough percentage of a population becomes immune—either through prior infection or vaccination—such that even the nonimmune are indirectly protected. To achieve population immunity for vaccine preventable diseases, high uptake rates are necessary. With COVID‐19’s estimated reproduction rate of 5.8, population immunity thresholds to stop COVID‐19 transmission range between 73% and 84%. 3 The potential for population immunity against COVID‐19 is threatened by hesitancy toward the COVID‐19 vaccine. Vaccine hesitancy refers to a spectrum of behaviors that include refusal as well as delayed acceptance of a vaccine. 4 Polls tracking COVID‐19 vaccine hesitancy in May of 2020 estimated that 28% of the US population would probably or definitely not get the COVID‐19 vaccine. 5 Hesitancy rose to between 49% and 50% in September 2020, and slightly declined to 40% and 42% in November 2020, the month before a vaccine would become available for the first time. 5 , 6
Prior research has demonstrated that sociodemographic factors, such as age, sex, race/ethnicity, income, and education are correlates of vaccine hesitancy. 7 , 8 Sociodemographic factors are understood to be dimensions of social positioning shaping life chances and experiences, subsequently influencing trust and assessment of risk. Vaccine hesitancy varies by race and ethnicity, with Black/African Americans reporting some of the highest level of general vaccine hesitancy and lowest levels of vaccine confidence. 9 , 10 The high level of vaccine hesitancy would be concerning on its own but combined with the growing evidence that COVID‐19 is exacting a disproportionate toll on racial minorities, it is especially worrisome. 2 , 11
Vaccine refusal and under‐vaccination at the community level is associated with resurgence of vaccine‐preventable diseases, including measles and pertussis. 12 Clusters of outbreaks occur in communities where vaccine uptake is not sufficient, 13 and geographic variation in COVID‐19 vaccine hesitancy suggests state‐level analysis may be equally as important as national‐level studies. 14 This paper examines vaccine hesitancy among adults living, working, or receiving care in Arkansas. Arkansas is in the top 10 states for rural populations (43.84%), which often lack adequate health care resources and primary care providers, 15 and is ranked third among US states with the highest percentages (46.50%) of populations at high risk for serious illness due to COVID‐19. 16 The following research questions were examined: (1) What proportion of Arkansans are hesitant to receive a vaccine for COVID‐19?; (2) How does COVID‐19 vaccine hesitancy differ across sociodemographic groups in Arkansas?; and (3) How does knowledge of how to protect one’s self against the virus (COVID‐19 health literacy), fear of the virus, and general trust for vaccines relate to COVID‐19 vaccine hesitancy? We propose the following series of hypotheses.
COVID‐19 vaccine hesitancy will be significantly associated with sociodemographic factors (H1.1: age; H1.2: sex; H1.3: race/ethnicity; H1.4: income; and H1.5: education).
COVID‐19 vaccine hesitancy will be negatively associated with COVID‐19 health literacy.
COVID‐19 vaccine hesitancy will be negatively associated with fear of COVID‐19 infection.
COVID‐19 vaccine hesitancy will be negatively associated with general vaccine confidence.
MATERIALS AND METHODS
Study design and respondents.
Data were collected via online survey from volunteer research participants enrolled in the ARresearch registry. 17 The survey was conducted in July and August 2020. Recruitment emails were distributed to 4077 valid email addresses. The emails described the study and invited individuals to complete the survey. Research Electronic Data Capture (REDCap) was used to administer the consent and survey. 18 Inclusion criteria included being an adult (age ≥18) and living, working, and/or receiving health care in the state of Arkansas. A total of 1288 responses to the survey were collected (response rate = 31.59%). Of those, 1221 met the inclusion criteria, and 1205 answered at least a portion of the survey. A $20 gift card was provided as an incentive. The study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences (UAMS; IRB #261226).
Measurement
COVID‐19 vaccine hesitancy was captured through the question “If a vaccine for COVID‐19 were available today, what is the likelihood that you would get vaccinated?” Possible responses included extremely likely, likely, unlikely, and very unlikely. Those responses were dichotomized to indicate vaccine hesitancy, with both “unlikely” responses coded as 1 and both “likely” responses coded as 0.
Sociodemographic measures included age, sex, race and ethnicity, income, and education. Age was measured as a continuous variable. Sex was measured as a nominal variable (i.e., men and women), as were race and ethnicity (i.e., Black/African American, White, Other racial groups, and Hispanic/Latinx people of all races). Income was measured in 4 ranked categories beginning with less than $25,000, $25,000 to less than $50,000, $50,000 to less than $75,000, and $75,000 or more. Education was a categorical variable and included high school diploma or less, some college or technical degree, and a 4‐year college degree or more. Nominal and ordinal measures were recoded as dummy variables when they were included in the full information maximum likelihood logistic regressions.
COVID‐19 health literacy was captured by the statement: “I know how to protect myself from COVID‐19.” Respondents could answer with “not sure at all,” “maybe/not sure,” and “yes, completely sure.” Less than 2% answered with “not sure at all,” so this response was combined with the “maybe/not sure” response and coded as 0 to indicate less health literacy. Those who answered “yes, completely sure” were coded as 1 to indicate higher health literacy regarding how to protect one’s self against COVID‐19.
Fear of COVID‐19 infection was measured by asking respondents to “rate the extent of your concern” about being infected by COVID‐19. Possible responses included “to a great extent,” “somewhat,” “very little,” and “not at all.” These were coded from 1 to 4, respectively, with the higher numbers indicating greater complacency toward COVID‐19 infection. Responses of “don’t know” or “prefer not to answer” were coded as missing ( n = 6).
General vaccine trust was assessed by asking, “Overall how much do you trust vaccines?” Respondents could answer with “not at all,” “very little,” “somewhat,” “to a great extent,” and “completely.” Those who answered “to a great extent” or “completely” were coded as 1 to indicate a high level of trust. The other responses were coded as 0 to indicate lower levels of trust.
Statistical analysis
We used bivariate analyses and multivariable logistic regressions to test associations among sociodemographic characteristics, COVID‐19 health literacy, fear of COVID‐19 infection, trust in vaccines, and COVID‐19 vaccine hesitancy. Bivariate relationships were tested using both t ‐tests and χ 2 analyses. Analyses were conducted using Stata/SE 15.1. We explored multivariable relationships among hesitancy about the COVID‐19 vaccine and sociodemographic characteristics, COVID‐19 health literacy, fear of COVID‐19 infection, and trust in vaccines. To minimize the limitations of missing data, we tested these associations in full information maximum likelihood (FIML) logistic regression analyses using Mplus version 7.8. 19 FIML logistic regression makes use of all nonmissing values in estimating model parameters and yields results comparable to modern missing data methods, such as multiple imputation. 19
Table Table1 1 presents the descriptive statistics for the sample. Respondents’ mean age was 48 years, and 75.23% were women. The sample closely resembled the racial composition of Arkansas and was distributed evenly across income categories, with each category making up between 21% and 27% of the sample. The sample was over‐representative of respondents age 25 years or older with a college degree (61.75%) compared to the state of Arkansas (23%) and the general US population (32%). 20 The majority (65.53%) of respondents felt completely sure they knew how to protect themselves against COVID‐19, and 72.03% reported high trust in vaccines in general. More than three‐quarters os the respondents (78.14%) reported that they were likely or extremely likely to get the COVID‐19 vaccine if it were available to them; however, 21.86% reported that they were unlikely or very unlikely to get it.
Descriptive statistics for sample of Arkansas adults
| Frequency | % or | SD | Range | |
|---|---|---|---|---|
| Sociodemographic controls | ||||
| Age | 1205 | 48.13 | 15.56 | 18.3–92.4 |
| Sex | 1203 | 0.75 | 0.43 | 0–1 |
| Women | 905 | 75.23 | ||
| Men | 298 | 24.77 | ||
| Race/ethnicity | 1202 | 2.03 | 0.65 | 1–4 |
| Black/African American | 161 | 13.39 | ||
| White | 918 | 76.37 | ||
| Other racial groups | 43 | 3.58 | ||
| Hispanic/Latinx | 80 | 6.66 | ||
| Income | 974 | 1.56 | 1.10 | 0–3 |
| <$25K | 205 | 21.05 | ||
| $25K <$50K | 284 | 29.16 | ||
| $50K <$75K | 221 | 22.69 | ||
| >$75K | 264 | 27.10 | ||
| Education | 1202 | 1.48 | 0.70 | 0–2 |
| High school or less | 145 | 12.06 | ||
| Some college | 331 | 27.54 | ||
| Four‐year degree | 726 | 60.40 | ||
| COVID‐19 attitudes/feelings | ||||
| COVID‐19 health literacy | 1082 | 0.345 | 0.47 | 0–1 |
| Maybe/not sure | 373 | 34.47 | ||
| Completely sure | 709 | 65.53 | ||
| Fear of COVID‐19 infection | 1069 | 2.05 | 0.80 | 1–4 |
| Great extent | 268 | 25.07 | ||
| Somewhat | 532 | 49.77 | ||
| Very little | 216 | 20.21 | ||
| Not at all | 53 | 4.96 | ||
| Vaccine confidence | ||||
| General vaccine trust | 1144 | 0.720 | 0.44 | 0–1 |
| Low trust | 320 | 27.97 | ||
| High trust | 824 | 72.03 | ||
| Vaccine hesitancy | ||||
| COVID‐19 vaccine hesitancy | 1066 | 0.218 | 0.41 | 0–1 |
| Hesitant | 233 | 21.86 | ||
| Not hesitant | 833 | 78.14 | ||
Abbreviation: COVID‐19, coronavirus disease 2019; SD, standard deviation.
Bivariate analyses
Table Table2 2 presents the bivariate analyses among all independent variables and COVID‐19 vaccine hesitancy. There was statistically significant variation in COVID‐19 vaccine hesitancy based on age ( t = 3.17; p < 0.01), race/ethnicity ( χ 2 = 68.93, p < 0.001), income ( χ 2 = 20.42, p < 0.001), education ( χ 2 = 32.04, p < 0.001), fear of infection ( χ 2 = 55.76, p < 0.001), and general vaccine confidence ( χ 2 = 257.01, p < 0.001). The average age for respondents reporting they were likely to get the COVID‐19 vaccine (49.02) was 3.66 years higher than the average for those who reported hesitancy (45.36). COVID‐19 vaccine hesitancy was highest among Black/African American respondents (50.00%), followed by Hispanic/Latinx respondents (19.18%), and White respondents (18.37%). COVID‐19 vaccine hesitancy was highest for those in the lowest income category (30.68%) and steadily declined as income category increased, with the highest income group reporting the lowest hesitancy (13.10%). Respondents with some college or a technical degree reported the highest prevalence of COVID‐19 vaccine hesitancy (32.17%) across education categories, followed by those with a high school degree or less (27.20%) and those with a 4‐year degree (16.23%) who reported the lowest prevalence of hesitancy. Respondents who did not fear infection of COVID‐19 at all had the highest prevalence of COVID‐19 vaccine hesitancy of any group (62.50%). Respondents who feared COVID‐19 infection to a great extent had a much lower prevalence of COVID‐19 vaccine hesitancy (15.90%). The greatest difference in COVID‐19 vaccine hesitancy was between respondents who have high trust (9.51%) and those who have low trust (55.84%) in vaccines in general. There was no significant difference in vaccine hesitancy by sex or COVID‐19 health literacy.
Prevalence of COVID‐19 vaccine hesitancy among Arkansans adults
| COVID‐19 vaccine hesitancy | ||
|---|---|---|
| Hesitant % ( ) or | ‐test or value | |
| Sociodemographic controls | ||
| Age | 45.36 | 0.002 |
| Sex | ||
| Women | 23.10 (182) | |
| Men | 18.41 (51) | |
| Race/ethnicity | < 0.001 | |
| Black/African American | 50.00 (64) | |
| White | 18.37 (151) | |
| Other racial groups | 9.76 (4) | |
| Hispanic/Latinx | 19.18 (14) | |
| Income | < 0.001 | |
| <$25K | 30.68 (54) | |
| $25K <$50K | 23.28 (61) | |
| $50K <$75K | 19.70 (40) | |
| >$75K | 13.10 (33) | |
| Education | < 0.001 | |
| High school or less | 27.20 (34) | |
| Some college | 32.17 (92) | |
| Four‐year degree | 16.23 (106) | |
| COVID‐19 attitudes/feelings | ||
| COVID‐19 health literacy | 0.574 | |
| Maybe/not sure | 20.30 (68) | |
| Completely sure | 21.85 (142) | |
| Fear of COVID‐19 infection | < .001 | |
| Great extent | 15.90 (38) | |
| Somewhat | 18.81 (92) | |
| Very little | 25.00 (49) | |
| Not at all | 62.50 (30) | |
| Vaccine confidence | ||
| General vaccine trust | < 0.001 | |
| Low trust | 55.84 (153) | |
| High trust | 9.51 (74) | |
Abbreviation: COVID‐19, coronavirus disease 2019.
Multivariable analyses
Table Table3 3 presents the FIML logistic regression results for vaccine hesitancy specific to the COVID‐19 vaccine. Odds of reporting COVID‐19 vaccine hesitancy were significantly associated with age, race/ethnicity, education, fear of infection, and general vaccine trust. Odds of COVID‐19 vaccine hesitancy decreased as age increased ( p < 0.05). Black/African American respondents had 2.42 times greater odds of vaccine hesitancy compared to White respondents ( p < 0.001). Those with some college or a technical degree had 1.67 times the odds of vaccine hesitancy compared to those with a 4‐year degree ( p < 0.05). Those who felt very little fear of COVID‐19 infection had 2.05 greater odds of COVID‐19 vaccine hesitancy compared to those who feared COVID‐19 infection to a great extent ( p < 0.05). Those who reported they did not fear a COVID‐19 infection at all had 5.48 greater odds of vaccine hesitancy compared to those who feared infection to a great extent ( p < 0.001). Those who had low trust in vaccines in general had 11.32 greater odds of vaccine hesitancy compared to those who reported low levels of trust ( p < 0.001).
FIML logistic regression of COVID‐19 vaccine hesitancy
| Est. | OR | SE | value | Sig. | |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age | −0.01 | 0.98 | 0.01 | 0.026 | * |
| Sex | |||||
| Female | 0.32 | 1.38 | 0.21 | 0.137 | |
| Race/ethnicity | |||||
| White | – | – | – | – | |
| Black/African American | 0.88 | 2.42 | 0.25 | < 0.001 | |
| Other racial groups | ‐1.26 | 0.28 | 0.64 | 0.049 | * |
| Hispanic/Latinx | −0.34 | 0.70 | 0.42 | 0.416 | |
| Income | |||||
| <$25K | 0.42 | 1.53 | 0.33 | 0.206 | |
| $25K <$50K | 0.21 | 1.24 | 0.30 | 0.476 | |
| $50K <$75K | 0.35 | 1.42 | 0.31 | 0.254 | |
| >$75K | – | – | – | – | |
| Education | |||||
| High school or less | −0.17 | 0.84 | 0.28 | 0.553 | |
| Some college | 0.51 | 1.67 | 0.23 | 0.028 | * |
| Four‐year degree | – | – | – | – | |
| COVID‐19 attitudes/feelings | |||||
| COVID‐19 health literacy | |||||
| Maybe/not sure | −0.42 | 0.65 | 0.21 | 0.050 | |
| Completely sure | – | – | – | – | |
| Fear of COVID‐19 infection | |||||
| Great extent | – | – | – | – | |
| Somewhat | 0.44 | 1.56 | 0.25 | 0.083 | |
| Very little | 0.72 | 2.05 | 0.29 | 0.014 | * |
| Not at all | 1.70 | 5.48 | 0.34 | < 0.001 | |
| Vaccine confidence | |||||
| General vaccine trust | |||||
| Low trust | 2.47 | 11.32 | 0.19 | < 0.001 | |
| High trust | – | – | – | – | |
Abbreviations: COVID‐19, coronavirus disease 2019; Est., Estimate; FIML, full information maximum likelihood; OR, odds ratio; SE, standard error; Sig., significance.
*** p < 0.001, * p < 0.05.
Overall, we find the majority of respondents (79%) are not hesitant to take a COVID‐19 vaccine. However, 1 in 5 (21.86%) did report vaccine hesitancy, selecting they would be unlikely or very unlikely to take a COVID‐19 vaccine. This is a large enough portion of the sample to potentially affect the capacity of the COVID‐19 vaccine to achieve population immunity (Ke et al., 2020). This is slightly lower than earlier estimates for COVID‐19 vaccine hesitancy in the US general population in May 2020 ranging from 27–33%, 5 , 14 but much lower than subsequent US estimates reported in September (49%) and November (39–44%) 2020. 5 , 6 , 21 The lower reported hesitancy may be due to changes in respondents’ views of the vaccine over time, differences in state versus national‐level hesitancy, the high percentage of those with 4‐year college degrees in this sample, or the increasing probability respondents or someone they know have been severely affected by COVID‐19.
COVID‐19 vaccine hesitancy differed significantly across age (H1.1), race/ethnicity (H1.3), income (H1.4), and education (H1.5). Respondents who were younger, Black/African American, lower income, and had some college or a technical degree had a higher prevalence and odds of vaccine hesitancy than those who were older, White, in higher income brackets, or 4‐year college degree holders. These findings are consistent with prior literature 14 , 22 , 23 but add new insight as the first article to have a large and diverse sample from a rural state with a large high‐risk population.
Half of all Black/African American respondents reported hesitancy to get the COVID‐19 vaccine, and their odds of vaccine hesitancy were more than double White respondents’. This finding is consistent with prior literature that documented Black/African American hesitancy towards the COVID‐19 vaccine. 14 , 21 , 22 , 23 This finding is of great concern because Black/African Americans bear a greater burden of COVID‐19 hospitalizations and deaths, 2 , 22 and hesitancy to get the COVID‐19 vaccine could perpetuate long‐term racial health disparities.
Racial differences in COVID‐19 vaccine attitudes highlight the need for “trustworthiness before trust.” 24 Distrust of the medical establishment by Black/African Americans is often traced back to the Tuskegee syphilis study, but the distrust is deeply rooted beyond a single incident and is predicated on centuries of racist exploitation by medical researchers and doctors. 25 Racism within the medical establishment is ongoing, and Black/African Americans do not need an extensive knowledge of the history of medical racism to inform their view of vaccines when many only need to consider recent experiences. 26 , 27 Ongoing and uncritical usage of terms such as “herd immunity” during this pandemic exemplifies the durability of language that intersected with eugenics and became popularized at a time when eugenic racism was growing in the (US) and United Kingdom. 28
As hypothesized, education was significantly associated with COVID‐19 vaccine hesitancy, which is partially consistent with prior studies. 14 , 22 , 23 However, we were surprised to find those with some college or a technical degree reported the highest prevalence of COVID‐19 vaccine hesitancy (32.17%) across the education categories, followed by those with a high school degree or less (27.20%) and those with a 4‐year degree (16.23%), who reported the lowest prevalence of vaccine hesitancy. Although some polls have demonstrated differences in hesitancy across educational attainment, results showed those with some college to be similar to those with high school education. 5 This study documents a more complex relationship between education and COVID‐19 vaccine hesitancy that should be examined further.
Vaccine hesitancy was positively associated with less fear of infection by COVID‐19 (H3). Specifically, those who feared COVID‐19 infection very little or not at all had odds of vaccine hesitancy two to five times greater than those who feared infection to a great extent. Black/African American respondents in this study had the highest prevalence of fearing infection to a great extent across all racial/ethnic groups, meaning that high COVID‐19 vaccine hesitancy for Black/African Americans is unlikely to be explained by insufficient fear of COVID‐19 infection. Our finding that a lack of or limited fear is significantly associated with vaccine hesitancy is consistent with prior studies on vaccines in general. 29 However, this is the first study to our knowledge to document a significant association between low levels of fear of infection and hesitancy to get the COVID‐19 vaccine.
As hypothesized, COVID‐19 vaccine hesitancy was negatively associated with general vaccine confidence (H4). Respondents who reported high levels of trust in vaccines in general had significantly lower odds of COVID‐19 vaccine hesitancy compared to those with low trust in vaccines in general. This finding is consistent with numerous findings on the relationship between trust and vaccine hesitancy, particularly those that examine racial/ethnic differences. 10 , 29 However, this study is among a limited body of work to examine general vaccine trust and hesitancy specific to COVID‐19 vaccines. We do not find support for differences in COVID‐19 vaccine hesitancy by sex (H1.2) or COVID‐19 health literacy (H2).
Strengths and limitations
This study does have limitations. The data are cross‐sectional and do not allow us to determine causality or assess trends in COVID‐19 vaccine hesitancy across time. The sample was over‐representative of women and college educated respondents, and the study recruited respondents who had previously enrolled in a registry to be contacted for research purposes. The high number of respondents with a college degree limits the generalizability of these results to the state population. Finally, our measure of health literacy is limited to the respondents’ own self‐assessment of how well they know how to protect themselves against COVID‐19, and we do not know if that assessment is accurate.
These limitations are offset to a degree by the large and diverse sample, which closely mirrored the racial/ethnic composition of a highly rural state. Another strength of this study is that it includes a large percentage of non‐metro residents (33.28%) as determined by rural‐urban commuting area (RUCA). Although examining racial/ethnic differences in vaccine hesitancy was a focus of this study, the limited number of responses from racial groups such as Native Hawaiians and Pacific Islanders, Asians, American Indian, and Alaskan Natives did not allow us to assess differences between some communities of color. Future studies of COVID‐19 vaccine hesitancy should consider oversampling for these groups to ensure specific information about their levels of vaccine hesitancy are known.
These results have already helped shape our vaccination efforts. The results of this study have been used to develop an equitable distribution plan within the state’s only academic medical center with specific targets to ensure racial/ethnic geographic and equality. Specifically, the authors and their institution have set up a mobile vaccine distribution system that goes into rural and remote areas and is partnering with community‐based and faith‐based organizations on a nontraditional educational campaign to build trust.
Understanding determinants of COVID‐19 vaccine hesitancy is important in ensuring broad uptake of the COVID‐19 vaccine and in reducing health disparities. Trust in vaccines in general, race/ethnicity, and fear of COVID‐19 infection are important factors shaping COVID‐19 vaccine hesitancy, with Black/African Americans reporting significantly more vaccine hesitancy. As Warren and colleagues 24 point out, trustworthiness must precede trust, and the historical and ongoing racism within medical institutions precludes trust. Building trust in institutions takes a concerted effort and time. Although the urgency of the pandemic is spurring efforts toward trust and transparency, without a sustained effort of community engagement, any gains in trust may be lost.
Vaccine hesitancy is high enough to undermine population immunity in a highly vulnerable and rural state in the US. Moreover, it is highest among the sociodemographic groups who have faced disproportionate COVID‐19 morbidity and mortality. Racial/ethnic disparities highlight historical and contemporary distrust in the medical establishment and ongoing experiences of racism/discrimination by communities of color. Finally, public health messaging for the COVID‐19 vaccine must consider the role of people’s fears of infection, general trust in vaccines, and the historical and ongoing mistreatment of many racial/ethnic minorities.
Further investigation is needed to determine ways in which fear and trust may or may not explain vaccine hesitancy across racial groups. The mechanisms explaining vaccine hesitancy are likely to differ across racial groups. For example, even though low levels of fear are associated with increased odds of vaccine hesitancy, Black/African Americans have both the highest levels of COVID‐19 vaccine hesitancy and the highest prevalence of fearing infection to a great extent. Moreover, given the consistent findings of racial disparities in vaccine hesitancy, researchers must begin to more explicitly examine experiences of discrimination and systemic racism in predicting vaccine hesitancy.
CONFLICT OF INTEREST
The authors declare no competing interests for this work.
AUTHOR CONTRIBUTIONS
D.E.W., J.A.A., K.B., J.P.S., C.R.L., H.C.F., G.M.C., and P.A.M. wrote the manuscript. D.E.W., J.A.A., J.P.S., and P.A.M. designed the research. D.E.W., J.A.A., J.P.S., and P.A.M. performed the research. D.E.W., J.A.A., and J.P.S. analyzed the data.
ACKNOWLEDGEMENTS
The study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences (UAMS) (IRB#261226).
Willis DE, Andersen JA, Bryant‐Moore K, et al. COVID‐19 vaccine hesitancy: Race/ethnicity, trust, and fear . Clin Transl Sci . 2021; 14 :2200–2207. 10.1111/cts.13077 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
The research described was supported by the Translational Research Institute (TRI), grant UL1 TR003107 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- Search Menu
- Sign in through your institution
- Advance articles
- Browse content in Biological, Health, and Medical Sciences
- Administration Of Health Services, Education, and Research
- Agricultural Sciences
- Allied Health Professions
- Anesthesiology
- Anthropology
- Anthropology (Biological, Health, and Medical Sciences)
- Applied Biological Sciences
- Biochemistry
- Biophysics and Computational Biology (Biological, Health, and Medical Sciences)
- Biostatistics
- Cell Biology
- Dermatology
- Developmental Biology
- Emergency Medicine
- Environmental Sciences (Biological, Health, and Medical Sciences)
- Immunology and Inflammation
- Internal Medicine
- Medical Sciences
- Medical Microbiology
- Microbiology
- Neuroscience
- Obstetrics and Gynecology
- Ophthalmology
- Pharmacology
- Physical Medicine
- Plant Biology
- Population Biology
- Psychological and Cognitive Sciences (Biological, Health, and Medical Sciences)
- Public Health and Epidemiology
- Radiation Oncology
- Rehabilitation
- Sustainability Science (Biological, Health, and Medical Sciences)
- Systems Biology
- Veterinary Medicine
- Browse content in Physical Sciences and Engineering
- Aerospace Engineering
- Applied Mathematics
- Applied Physical Sciences
- Bioengineering
- Biophysics and Computational Biology (Physical Sciences and Engineering)
- Chemical Engineering
- Civil and Environmental Engineering
- Computer Sciences
- Computer Science and Engineering
- Earth Resources Engineering
- Earth, Atmospheric, and Planetary Sciences
- Electric Power and Energy Systems Engineering
- Electronics, Communications and Information Systems Engineering
- Engineering
- Environmental Sciences (Physical Sciences and Engineering)
- Materials Engineering
- Mathematics
- Mechanical Engineering
- Sustainability Science (Physical Sciences and Engineering)
- Browse content in Social and Political Sciences
- Anthropology (Social and Political Sciences)
- Economic Sciences
- Environmental Sciences (Social and Political Sciences)
- Political Sciences
- Psychological and Cognitive Sciences (Social and Political Sciences)
- Social Sciences
- Sustainability Science (Social and Political Sciences)
- Author guidelines
- Submission site
- Open access policy
- Self-archiving policy
- Why submit to PNAS Nexus
- The PNAS portfolio
- For reviewers
- About PNAS Nexus
- About National Academy of Sciences
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, materials and methods, discussion and limitations, acknowledgments, supplementary material, author contributions, data availability.
- < Previous
Spatial scales of COVID-19 transmission in Mexico
Competing Interest: The authors declare no competing interest.
- Article contents
- Figures & tables
- Supplementary Data
Brennan Klein, Harrison Hartle, Munik Shrestha, Ana Cecilia Zenteno, David Barros Sierra Cordera, José R Nicolás-Carlock, Ana I Bento, Benjamin M Althouse, Bernardo Gutierrez, Marina Escalera-Zamudio, Arturo Reyes-Sandoval, Oliver G Pybus, Alessandro Vespignani, José Alberto Díaz-Quiñonez, Samuel V Scarpino, Moritz U G Kraemer, Spatial scales of COVID-19 transmission in Mexico, PNAS Nexus , Volume 3, Issue 9, September 2024, pgae306, https://doi.org/10.1093/pnasnexus/pgae306
- Permissions Icon Permissions
During outbreaks of emerging infectious diseases, internationally connected cities often experience large and early outbreaks, while rural regions follow after some delay. This hierarchical structure of disease spread is influenced primarily by the multiscale structure of human mobility. However, during the COVID-19 epidemic, public health responses typically did not take into consideration the explicit spatial structure of human mobility when designing nonpharmaceutical interventions (NPIs). NPIs were applied primarily at national or regional scales. Here, we use weekly anonymized and aggregated human mobility data and spatially highly resolved data on COVID-19 cases at the municipality level in Mexico to investigate how behavioral changes in response to the pandemic have altered the spatial scales of transmission and interventions during its first wave (March–June 2020). We find that the epidemic dynamics in Mexico were initially driven by exports of COVID-19 cases from Mexico State and Mexico City, where early outbreaks occurred. The mobility network shifted after the implementation of interventions in late March 2020, and the mobility network communities became more disjointed while epidemics in these communities became increasingly synchronized. Our results provide dynamic insights into how to use network science and epidemiological modeling to inform the spatial scale at which interventions are most impactful in mitigating the spread of COVID-19 and infectious diseases in general.
Outbreaks of infectious diseases, including COVID-19 across different localities are linked via human mobility. Using aggregated human mobility data at the municipality level in Mexico, we find that scales of human mixing and predictability of COVID-19 growth rates shift during the pandemic which improves the ability to target spatial interventions. These dynamical insights are useful when preparing for new outbreaks and planning disease surveillance using a combination of digital mobility and epidemiological data.
The transmission of infectious diseases is highly heterogeneous. Differences in population structure, the landscape of immunity, and environmental factors, result in differences in the timing of outbreaks, their magnitude, and duration ( 1–15 ). For infectious diseases, one principal component determining the spatial structure of outbreaks is the frequency of interactions between susceptible and infectious individuals within and between regions ( 6 , 9 , 16–19 ). In most geographies, public health decision-making authority follows political/administrative boundaries ( 20–26 ). However, from an epidemiological perspective, the relevant spatial units may not strictly follow political boundaries but rather human mixing ( 1 , 3 , 27 ). Evaluating the spatial structure of COVID-19 transmission remains important in determining optimal interventions (nonpharmaceutical and/or vaccination) to reduce transmission and limit the risk of resurgence of cases ( 28–31 ). Whereas prior work has been focused on determining the synchrony of epidemics across spatial units ( 32–34 ) we extend that work by investigating how epidemic synchrony varies by mobility informed spatial aggregations.
During the first half of 2020, Mexico experienced one of the largest SARS-CoV-2 epidemics worldwide, with more than 600,000 cases (Fig. 1 A,B) and 65,000 confirmed deaths reported between February and September 2020 ( 35 ). The epidemic wave peaked in late May in Mexico City (Ciudad de Mexico and formerly known as Distrito Federal) and later ignited epidemics in all other states ( 36 ), peaking between June and late July 2020 (Fig. 1 B). Mexico has a complex human mobility network with Mexico City playing a pivotal role in determining the dynamics of respiratory infections ( 37–40 ). Here, we combine municipality level epidemiological data with weekly anonymized aggregated human mobility data at the same scale, to characterize the spatial scales of the Mexican COVID-19 pandemic and their implications for the implementation of spatially targeted interventions. We investigate whether grouping municipalities by their mobility patterns as opposed to administrative units yields more meaningful epidemiological predictions.

Epidemiological situation of COVID-19 in Mexico. A) Map of cumulative cases per 100,000 people, as of 2020 September 1. B) Timeline of new cases per 100,000 population at the state level (7-day rolling average), highlighting the 15 states with the most severe cumulative outbreaks. C) Number of municipalities that reported confirmed cases of COVID-19 through time. D) Age and sex distributions of confirmed COVID-19 cases across Mexico, highlighting “early” and “late” periods during which the relative risk of infections were calculated. E) Age and sex relative risk ratios of infection, comparing the early vs. late periods from (D).
Epidemiological data
Epidemiological data include individual-level information on patients with confirmed RTq-PCR COVID-19 infection between 2020 March–September 30th. Data were downloaded from http://datosabiertos.salud.gob.mx/gobmx/salud/datos_abiertos/datos_abiertos_covid19.zip (last accessed 2020 October 24). Data include information about patients demographics (age and sex) and municipality of residence. In all analyses, we used the date of onset of symptoms.
Population and travel data
Human mobility and population data were extracted at the municipality level based on the 2016 boundaries (INEGI 2016: https://www.inegi.org.mx/app/mapa/espacioydatos/default.aspx ). Population data were downloaded from the COVID-19 indicator dataset, which was provided by INEGI ( https://www.inegi.org.mx/investigacion/covid/ ).
Aggregated and anonymized human mobility data
We used the Google COVID-19 Aggregated Mobility Research Dataset described in detail in Refs. ( 41 , 42 ), which contains anonymized relative mobility flows aggregated over users who have turned on the Location History setting, which is turned off by default. This is similar to the data used to show how busy certain types of places are in Google Maps—helping identify when a local business tends to be the most crowded. The mobility flux is aggregated per week, between pairs of approximately 5 km 2 cells worldwide, and for the purpose of this study further aggregated for municipalities in Mexico.
To produce this dataset, machine learning is applied to log data to automatically segment it into semantic trips. To provide strong privacy guarantees ( 43 ), all trips were anonymized and aggregated using a differentially private mechanism to aggregate flows over time (see https://policies.google.com/technologies/anonymization ). This research is done on the resulting heavily aggregated and differentially private data. No individual user data was ever manually inspected, only heavily aggregated flows of large populations were handled. All anonymized trips are processed in aggregate to extract their origin and destination location and time. For example, if n users traveled from location a to location b within time interval t , the corresponding cell ( a , b , t ) in the tensor would be n ± e r r , where err is Laplacian noise. The automated Laplace mechanism adds random noise drawn from a zero mean Laplacian distribution and yields ( ϵ , δ ) -differential privacy guarantee of ϵ = 0.66 and δ = 2.1 × 10 29 per metric. Specifically, for each week W and each location pair ( A , B ) , we compute the number of unique users who took a trip from location A to location B during week W . To each of these metrics, we add Laplace noise from a zero-mean distribution of scale 1 / 0.66 . We then remove all metrics for which the noisy number of users is lower than 100, following the process described in Ref. ( 43 ), and publish the rest. This yields that each metric we publish satisfies ( ϵ , δ ) -differential privacy with values defined above. The parameter ϵ controls the noise intensity in terms of its variance, while δ represents the deviation from pure ϵ -privacy. The closer they are to zero, the stronger the privacy guarantees.
These results should be interpreted in light of several important limitations. First, the Google mobility data are limited to smartphone users who have opted into Google’s Location History feature, which is off by default. These data may not be representative of the population as whole, and furthermore their representativeness may vary by location. Importantly, these limited data are only viewed through the lens of differential privacy algorithms, specifically designed to protect user anonymity and obscure fine detail. Moreover, comparisons across rather than within locations are only descriptive since these regions can differ in substantial ways.
Timeline of interventions
The Mexican government has outlined four principle objectives for the control of COVID-19 (i) reduce risk of acquiring infection, (ii) reduce risk of severe morbidity and mortality, (iii) reduce risk and impact on society, and (iv) reduce risk of transmission between infectious and susceptible individuals. We collated a full list of interventions between February and September 2020 and details are provided in Table S1 , including references.
Relative risk model
Following Goldstein and Lipsitch ( 44 ), we used age stratified epidemiological data to assess the temporal shifts in the share of a given age group among all cases of infection. To do so, we use the relative risk (RR) ( 45 , 46 ) statistic that estimates the ratio of the proportion of a given age group among all detected cases of COVID-19 for a later time period vs. an early time period. We selected the early time period to be the month of April (the period right after the implementation of the lockdown) and the late period to be June to September. We adopted the code and model from Goldstein and Lipsitch described in detail ( 44 ).
Community detection algorithm
Human mobility networks, based on data from mobile devices, can be used to capture important population-level trends. Microscopic descriptions often remain too complex to extract meaningful information to describe the transmission process accurately ( 32 ). We here use a community detection algorithm following ( 47 ) to identify human movement communities (basins) where within-community mobility among municipalities is higher than across-community mobility. We chose this community detection algorithm as it is conceptually related to spatial infectious disease transmission models.
Municipality-level case growth rates
To estimate the daily epidemic growth rates in each municipality, we fit a mixed effects generalized linear model (GLM) of natural log new daily case counts in sliding 7-day windows (fixed effect; approximately the generation time of COVID-19 in the earliest wave) and a random effect for each municipality on the slope and intercept separately for each municipality, using the R package lme4 v.1.1-21 ( 48 ). Daily case counts were determined using the date of symptom onset. Below is the functional form of this regression model for a single municipality. We iterate over each municipality separately and store the resulting growth rate.
Relationship between case growth rates and mobility
To test for an effect of mobility from Mexico City on municipality growth rates, we fit a mixed effect GLM with log mobility between Mexico city and each municipality as a fixed effect, a random effect on the intercept for each municipality and a random effect on the slope and intercept for the log mobility each week. The conditional and marginal coefficient of determination, i.e. R 2 , were calculated using the R package MuMIn v1.471. ( 49 ) which implements the method developed by Nakagawa et al. ( 50 ). Model selection was performed using analysis of variance for mixed effects models as implemented in the R package lmerTest v.3.1-3 ( 51 ).
Spatial expansion of COVID-19 in Mexico
In Mexico, the spatial range of transmission expanded rapidly after reports of the earliest cases in March 2020, with over 700 municipalities reporting transmission by July 2020 (out of 2,448, Fig. 1 C). During April and May, the risk of positive RTq-PCR confirmed cases amongst men aged 30–69 was 1.4 times higher than between July 1 and September 1 (Fig. 1 D,E), indicating that the epidemic spread initially within and through these age groups (Fig. S2 ). This dynamic trend in the demographics of cases is similar to that observed in other countries during the early stages of the pandemic ( 53 , 54 ).
Mexico City experienced early and widespread cases of COVID-19 (Fig. 1 B) ( 36 ) and due to its centrality including with its surrounding state, Mexico State connecting people from abroad (international arrivals) and within Mexico we hypothesize that human mobility from these two states was a key driver of the spread of COVID-19 in Mexico. Using anonymized, opt-in and aggregated human movement data from mobile phones (see Materials and methods for a more detailed description of the mobility data) we find that case growth rates across Mexican states were associated with human movements from the State of Mexico and Mexico City between March and May 2020 (regression coefficient from generalized linear mixed effects model (fixed effect of municipality and log10 mobility between Mexico City and each municipality; random effects accounting for effects of time and municipality on the slope and intercept) shown in Fig. 2 C, conditional R 2 = 0.62 ; see Materials and methods for a detailed description of the statistical model). This pattern is analogous to outbreaks which were driven by major cities in the United Kingdom and China ( 53 , 55 ). Further, we observe that the share of overall relative human mobility to and from Mexico and Mexico City increased markedly during that period (Fig. 2 D) when overall human mobility between states declined (Figs. 2 B and S3 showing state-level data on change in human mobility). This points towards a change in the network structure of human mobility in Mexico, as documented in some other countries ( 56 , 57 ). Overall transmission, and the importance of Mexico City driving the epidemic, declined after the implementation of NPIs through May 2020. However, after the lifting of physical distancing measures on June 1st (see table of documented changes in NPIs, Table S1 ), case growth rates in the country increased again as a function of mobility from Mexico City, in line with models predicting that lifting lockdowns can lead to reseeding of transmission chains from larger to smaller cities where epidemics were successfully controlled (Fig. 2 B, Table S1 , ( 13 )).

Human mobility and transmission of COVID-19 in Mexico. A) Prepandemic average of the inter-municipality mobility network, colored by network community (detected using the Infomap algorithm). Mobility flow data are based on the aggregated Google Mobility Research dataset (see Materials and methods). B) Deviation of weekly human mobility (number of flows within (grey line) and between states (black line)) from baseline (baseline mobility is calculated as the mean weekly mobility between 2020 January 12 and February 29). C) Evolution of the coefficients of mobility flow from Mexico City in (lagged) correlations with state-level case rates across the country, highlighting the key role that mobility from Mexico City played in the early stage of the epidemic. D) Average fraction of total outgoing mobility from each state that is to Mexico City (black) and the median entropy of states’ distributions of outgoing mobility. Error bands correspond to 95% confidence intervals.
Variation in weekly new cases within each state in Mexico are generally well predicted by cases in Mexico City weighted by human mobility except for Baja California, Morelos, Chihuahua, Oaxaca, and Chiapas (Fig. S4 ). We hypothesize that epidemics there were possibly seeded from other countries (United States and Guatemala); further SARS-CoV-2 genomic analyses of unbiased collections of samples will be needed to confirm the SARS-CoV-2 lineage dynamics in these states ( 34 , 36 , 55 , 58–60 ). Human mobility data showing cross border (United States to Mexico) movements indicate higher overall mobility to bordering states in Mexico and growth rates in United States–Mexico border states appear higher in the period between May 24–June 28, 2020 (Figs. S5, S6, and S7 ). The high degree of mobility during that phase resulted in larger case numbers and earlier peaks in states bordering the United States when compared to other states in Mexico (Fig. S6 ).
The scales of COVID-19 transmission
It is well known that reductions in mobility (a proxy for reductions in population mixing) have reduced the transmission of COVID-19 within a location especially during the early phase of a disease outbreak ( 61 , 62 ). However, it remains unclear how structural changes to the mobility network (shifts in the frequency and intensity of mobility within and among regions) have impacted COVID-19 dynamics empirically ( 56 , 57 , 63–65 ). Our underlying hypothesis is that more tightly connected communities exhibit more synchronized epidemic dynamics and, conversely, that more disjointed individual communities have less synchronized epidemics and their epidemics are more likely to fade out ( 16 , 18 , 19 ). We here refer to communities as the equivalent to municipalities and synchrony is defined as the similarity among communities in weekly case growth rates ( 66 )). Both processes have critical implications for disease mitigation and eliminations locally, and at a country level ( 13 , 67–71 ). The Mexican government announced stringent physical distancing policies on 2020 March 30th which resulted in marked changes in the mobility network (Table S1 , Fig. 2 B).
To quantify the degree to which mobility patterns are structured by geopolitical boundaries, we use a community detection algorithm that groups municipalities based on their movement patterns ( 47 ). Specifically, we aim to identify groups of municipalities such that movements between municipalities within the same group, i.e. community, are more frequent than movements to other municipalities in other communities. Community detection is often accomplished via modularity maximization ( 72 ); however, these approaches neglect information about the flow of mobility through the network. Instead, we leverage the map equation via an algorithm called InfoMap ( 47 ). The InfoMap algorithm utilizes an information theoretic approach to derive expected connectivity patterns if the observed flows were entirely determined by a random walk process. For this study, InfoMap is ideal because it is conceptually related to infectious disease transmission models, which often also utilize stochastic processes ( 73 ).
The aim is to identify municipalities where frequent interactions between individuals occur, such that the detected communities approximate the spatial scales of disease transmission (i.e. communities in which it is assumed that infection spreads via contacts within a relatively homogeneously mixing population ( 74 )). Accounting for spatial heterogeneity is known to be important for assessing strategies for interventions ( 6 ), especially in areas that have marked differences in urban and rural areas ( 75 ). Using this algorithm, we identify 16 mobility communities before the first cases of COVID-19 were detected in Mexico and consider this as the baseline (Fig. 2 A). Community size and organization changed following the announcement of the lockdown (2020 March 23 and 30) in Mexico and communities generally became smaller as compared to the pre-pandemic period (fewer municipalities within each community (Figs. S8 and S9 show the communities for each week during the study period). At the peak of the lockdown, we identified approximately 60 mobility communities (a 4-fold increase from the baseline period).
More specifically, there are two notable shifts in the network following the introduction of NPIs. First, more communities are identified but importantly the size of these communities shrinks disproportionately so that one community expands (Mexico City) and many very small ones emerge (Fig. 2 D). Further, as a result of the lockdown human movements across municipalities decline more rapidly than movements within a community with one important exception: Mexico City. There we observe that the ratio of within municipality movements declines at a similar rate than movements across municipalities (Fig. S3 ) further proving its central importance in the mobility network in Mexico.
We then compared the weekly infection incidence growth rates within each community and contrasted them to growth rates under a scenario in which municipalities are grouped based on state boundaries (black lines, Fig. 3 A,B). As expected, we find that epidemics in municipalities that are grouped by human mobility were more synchronized compared to those grouped by state. (Fig. 3 C; see Fig. S1 for a comparison of the within-state and within-community variability in municipality-level epidemic growth rates. Note that the variability is largest in the two states encompassing Mexico City, i.e. México and Distrito Federal, and much smaller in other states. The states encompasing Mexico City are grouped together in community 1, which is reflected in community 1’s much larger observed variability in municipality growth.) The synchrony among municipalities within each community were maximized in April and May 2020, a period when cases were rapidly rising across the country. After June, epidemics that are grouped by movement are still more synchronized, but the differences with groupings by state appear to be smaller (Fig. 3 C). This later period (June to October 2020) is a time when Mexico City appears to also lose importance in seeding the epidemic across the country, and local factors (e.g. population size) became more important in determining the epidemic trajectory ( 76 ). These results are expected as local factors become more influential in determining disease dynamics (population size, local mixing) and that the importance of continued virus re-importations wanes through time ( 55 ).

Network structure determines the synchrony of epidemics. A) Grouping of municipalities based on the state administrative boundaries. Gray shaded municipalities are removed from downstream analyses as they could not be assigned a movement community (see Materials and methods). B) Example grouping of municipalities based on human movement data and a community detection algorithm ( 52 ) (see Materials and methods). Colors indicate movement communities. Grey municipalities have limited recorded movements and could not be assigned to a community and were consequently excluded from analysis. C) Synchrony of weekly growth rates of epidemics across municipalities as measured by the pairwise standard error between growth rates. The lower the error, the more synchronized epidemics are. Blue line shows grouping by network communities, and orange shows groupings by state administrative boundaries. The green dashed line shows the nationwide trend in reported cases during this period. For comparison, please also see differences in within-state and withing-community standard deviations of growth rates in Fig. S1 .
We present a generalizable approach for understanding the spatial structure of transmission of COVID-19 and other emerging infectious diseases by accounting for the variations of the human mobility network that occurred as NPIs were implemented in Mexico. We aimed to differentiate the transmission dynamics at a level defined by administrative boundaries from that defined by simple community detection algorithms that are applied to aggregated anonymized weekly human mobility data. We find that as human mobility network structures change, so does the spatial transmission with implications for how interventions might be applied across municipalities identified as having synchronized epidemics. Because most NPIs are implemented around administrative boundaries—even within countries—incorporating these findings into real-world public health decision-making (specifically the coordination of NPIs across highly connected areas) may result in more effective strategies to control an epidemic in Mexico and elsewhere ( 77–80 ). The European Commission for example published a report on mobility functional areas (MFAs) which were informed by mobile phone data but the adoption of these recommendations remained sparse ( 79 ) and work based on data from the United States in mid-2020 found that a lack of NPI coordination across administrative boundaries could lead to unintended epidemiological consequences ( 81 ). Testing our framework beyond Mexico will be important for more coordinated action across countries.
Our model and results are only as accurate as the data that go into them. The Mexican COVID-19 database may suffer from underreporting due to testing shortages, changing case definitions and spatial heterogeneity in reporting ( 82 , 83 ). For example, relatively few cases were reported from Oaxaca (Fig. 1 A) which may be due to barriers to access to testing ( 84 ). Additionally, variable access to testing can influence observed epidemic growth rates, which is difficult to control for but unlikely to systematically bias our results on community structure ( 85 , 86 ). Future extensions of the model and as the pandemic continues will need to take into account high-resolution SARS-CoV-2 data on prior immunity to specific variants. Further, our model is based on higher level descriptions of the population (raw case data and population level human movement data) and these do not capture the high contact heterogeneity within each municipality (e.g. demographic heterogeneity and assortative mixing). These heterogeneities have been shown to be important in the clustering of transmission of COVID-19 with implications for targeted control ( 32 ). Contact patterns may differ significantly by age group, employment status and other factors not accounted for in this work. We did however observe heterogeneity in the demographic makeup of cases during the earlier phases of the Mexican COVID-19 pandemic.
Further, results should be interpreted in light of important limitations related to the human mobility data. First, the Google mobility data is limited to smartphone users who have opted into Google’s Location History feature, which is off by default. These data may not be representative of the population as whole, and furthermore their representativeness may vary by municipality. Importantly, these limited data are only viewed through the lens of differential privacy algorithms, specifically designed to protect user anonymity and obscure fine detail. Additionally, we used the most common form of the community detection algorithm InfoMap which might not be perfectly suited to represent human mobility patterns. Future work should be focused on developing and testing community detection algorithms that are tailored to the study of epidemics across scales ( 87–91 ).
Mexico is composed of 31 free and sovereign states and Mexico City, united under a federation. This means that each administrative region or state is governed by its own constitution, although they are not completely independent of the federal jurisdiction. Furthermore, each state is divided into municipalities, the nation’s basic administrative unit, which possesses limited autonomy (discretionary power on how best to respond to, or apply a public policy). Under a serious nationwide health threat or emergency, such as a pandemic, the federal Ministry of Health (MoH) acquires full authority over the health policies to be implemented nationwide. Nevertheless, Mexican law establishes that the General Health Council (GHC), a collegial body that reports to the president of the republic has the character of health authority, and can emit obligatory norms to be abided by the MoH. The GHC is presided by the Minister of Health and is conformed by federal institutions (e.g. Economy, Communication and Transport) as well as academic institutions, representatives from pharmaceutical industry, and other health system actors ( 92 ). Given its mandate and position in the Mexican health system, the GHC constitutes a promising agent to drive public policy outside of the margins or across geo-administrative units. Furthermore, there are examples of inter-state and inter-municipality coordination to resolve problems that extend beyond their borders such as waste management, tax, policing, and perhaps most relevant, health provision. It is in these contexts where evidence-based interventions on innovative approaches, such as the ones presented here become not only an option but a possibility, with greater impact in reducing transmission as compared to approaches where interventions are based on administrative boundaries.
However, theory often differs from practice and reality brings along additional and expected factors into play (e.g. economic ( 93 ) and political interests) many of which are not accounted for in this work. Some state governors for example refused to comply with federal health policies in the early relaxation phase in May 2020 ( 94 ). Future work focusing on the complex interplay between epidemiological vs. other considerations is important in translating our approach into public health policy.
Mexico has suffered a large and devastating epidemic, and we hope that our findings contribute to a more rational implementation of interventions in the future that can account for the substantial and changing spatial heterogeneity in transmission. Such analyses can be updated and translated to any other country in the world for which aggregated human mobility data are available. Future work should also focus on validating the inferred spatial scales with genomic data ( 55 , 60 , 95 ) or other coarse-graining techniques ( 96 , 97 ). Developing interventions using patterns observed in empirical mobility networks must be added to the list of priorities for pandemic response and preparedness in the 21st century.
We thank all health care workers and those involved in the collection, processing and publishing COVID-19 epidemiological data from Mexico.
Supplementary material is available at PNAS Nexus online.
M.U.G.K. acknowledges funding from The Rockefeller Foundation (PC-2022-POP-005), Google.org (also S.V.S.), the Oxford Martin School Pandemic Genomics (also O.G.P., B.G.) and Digital Pandemic Preparedness programmes, European Union’s Horizon Europe programme projects MOOD (#874850) and E4Warning (#101086640), the John Fell Fund, a Branco Weiss Fellowship, and Wellcome Trust grants 225288/Z/22/Z, 226052/Z/22/Z and 228186/Z/23/Z (also S.V.S.), United Kingdom Research and Innovation (#APP8583) and the Medical Research Foundation (MRF-RG-ICCH-2022-100069). B.K., H.H., S.V.S., and A.V. acknowledge the support of a grant from the John Templeton Foundation (61780) and the AccelNet-MultiNet program, a project of the National Science Foundation (Awards #1927425 and #1927418). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission, the National Science Foundation, The Rockefeller Foundation, the John Templeton Foundation, Google.org , the Wellcome Trust, nor any other funder.
S.V.S., M.U.G.K., and B.K. developed the idea, planned the research, and conducted analyses. A.C.Z. and D.B.S.C. collected government intervention data. S.V.S., M.U.G.K., and B.K. wrote the first draft of the manuscript. All authors interpreted the data, contributed to writing, and approved the manuscript.
A preprint of this article has been published online at https://arxiv.org/abs/2301.13256 .
The Google COVID-19 Aggregated Mobility Research Dataset used for this study is available with permission from Google LLC. Publicly available data and all code necessary to recreate the study are hosted on GitHub https://github.com/Emergent-Epidemics/COVID_Mexico and archived on Zenodo https://zenodo.org/doi/10.5281/zenodo.11372046 .
Alessandretti L , Aslak U , Lehmann S . 2020 . The scales of human mobility . Nature . 587 ( 7834 ): 402 – 407 .
Google Scholar
Burghardt K , Guo S , Lerman K . 2022 . Unequal impact and spatial aggregation distort COVID-19 growth rates . Philos Trans R Soc A . 380 ( 2214 ): 20210122 .
Lee EC , Arab A , Colizza V , Bansal S . 2022 . Spatial aggregation choice in the era of digital and administrative surveillance data . PLOS Digital Health . 1 ( 6 ): e0000039 .
Levin SA . 1992 . The problem of pattern and scale in ecology: the Robert H. Macarthur award lecture . Ecology . 73 ( 6 ): 1943 – 1967 .
Masters NB , et al . 2020 . Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data . Proc Natl Acad Sci U S A . 117 ( 45 ): 28506 – 28514 .
May RM , Anderson RM . 1984 . Spatial heterogeneity and the design of immunization programs . Math Biosci . 72 ( 1 ): 83 – 111 .
Perkins TA , Scott TW , Le Menach A , Smith DL . 2013 . Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission . PLoS Comput Biol . 9 ( 12 ): e1003327 .
Rader B , et al . 2020 . Crowding and the shape of COVID-19 epidemics . Nat Med . 26 ( 12 ): 1829 – 1834 .
Rice BL , et al . 2021 . Variation in SARS-CoV-2 outbreaks across sub-saharan Africa . Nat Med . 27 ( 3 ): 447 – 453 .
Rosensteel GE , Lee EC , Colizza V , Bansal S . 2021 . Characterizing an epidemiological geography of the United States: influenza as a case study. medRxiv 2021.02.24.21252361. 10.1101/2021.02.24.21252361 , preprint: not peer reviewed.
Smith DL . 2005 . Spatial heterogeneity in infectious disease epidemics. In: Lovett GM, Turner MG, Jones CG, Weathers KC, editors. Ecosystem function in heterogeneous landscapes . New York (NY): Springer. p. 137–164 .
Susswein Z , et al . 2021 . Ignoring spatial heterogeneity in drivers of SARS-CoV-2 transmission in the US will impede sustained elimination. medRxiv 2021.08.09.21261807. 10.1101/2021.08.09.21261807 , preprint: not peer reviewed.
Watts DJ , Muhamad R , Medina DC , Dodds PS . 2005 . Multiscale, resurgent epidemics in a hierarchical metapopulation model . Proc Natl Acad Sci U S A . 102 ( 32 ): 11157 – 11162 .
Wu SL , et al . 2023 . Spatial dynamics of malaria transmission . PLoS Comput Biol . 19 ( 6 ): e1010684 .
Yang W , et al . 2021 . Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis . Lancet Infect Dis . 21 ( 2 ): 203 – 212 .
Balcan D , et al . 2009 . Multiscale mobility networks and the spatial spreading of infectious diseases . Proc Natl Acad Sci U S A . 106 ( 51 ): 21484 – 21489 .
Brockmann D , Helbing D . 2013 . The hidden geometry of complex, network-driven contagion phenomena . Science . 342 ( 6164 ): 1337 – 1342 .
Davis JT , et al . 2021 . Cryptic transmission of SARS-CoV-2 and the first COVID-19 wave . Nature . 600 ( 7887 ): 127 – 132 .
Viboud C , et al . 2006 . Synchrony, waves, and spatial hierarchies in the spread of influenza . Science . 312 ( 5772 ): 447 – 451 .
Handfield RB , Patrucco AS , Wu Z , Yukins C , Slaughter T . 2024 . A new acquisition model for the next disaster: overcoming disaster federalism issues through effective utilization of the strategic national stockpile . Public Adm Rev . 84 ( 1 ): 65 – 85 .
Hildebrand S , Wehde W . 2023 . Examining factors associated with emergency managers’ collaborative planning with health departments prior to and during the covid-19 pandemic . Public Adm Rev . 83 ( 5 ): 1351 – 1366 .
Kettl DF . 2006 . Managing boundaries in American administration: the collaboration imperative . Public Adm Rev . 66 : 10 – 19 .
Landy M . 2008 . Mega-disasters and federalism . Public Adm Rev . 68 : S186 – S198 .
Merlin-Brogniart C , et al . 2022 . Social innovation and public service: a literature review of multi-actor collaborative approaches in five European countries . Technol Forecast Soc Change . 182 : 121826 .
Oliu-Barton M , Pradelski BSR . 2021 . Green zoning: an effective policy tool to tackle the COVID-19 pandemic . Health Policy (New York) . 125 ( 8 ): 981 – 986 .
Quick KS , Feldman MS . 2014 . Boundaries as junctures: collaborative boundary work for building efficient resilience . J Public Adm Res Theory . 24 ( 3 ): 673 – 695 .
Nelson GD , Rae A . 2016 . An economic geography of the United States: from commutes to megaregions . PLoS One . 11 ( 11 ): e0166083 .
Di Domenico L , Pullano G , Sabbatini CE , Boëlle P-Y , Colizza V . 2020 . Impact of lockdown on covid-19 epidemic in île-de-France and possible exit strategies . BMC Med . 18 ( 1 ): 240 .
Edsberg Møllgaard P , Lehmann S , Alessandretti L . 2022 . Understanding components of mobility during the COVID-19 pandemic . Philos Trans R Soc A . 380 ( 2214 ): 20210118 .
Graham M , et al . 2019 . Measles and the canonical path to elimination . Science . 364 ( 6440 ): 584 – 587 .
Lee EC , Wada NI , Grabowski MK , Gurley ES , Lessler J . 2020 . The engines of SARS-CoV-2 spread . Science . 370 ( 6515 ): 406 – 407 .
Chang S , et al . 2021 . Mobility network models of COVID-19 explain inequities and inform reopening . Nature . 589 ( 7840 ): 82 – 87 .
Dalziel BD , et al . 2018 . Urbanization and humidity shape the intensity of influenza epidemics in U.S. cities . Science . 362 ( 6410 ): 75 – 79 .
du Plessis L , et al . 2021 . Establishment and lineage dynamics of the SARS-CoV-2 epidemic in the UK . Science . 371 ( 6530 ): 708 – 712 .
GOB.MX. Coronavirus. https://coronavirus.gob.mx/
Taboada B , et al . 2020 . Genomic analysis of early SARS-CoV-2 variants introduced in Mexico . J Virol . 94 ( 18 ): e01056 – 20 .
Arieli Herrera-Valdez M , Cruz-Aponte M , Castillo-Chavez C . 2010 . Multiple outbreaks for the same pandemic: local transportation and social distancing explain the different “waves” of A-H1N1pdm cases observed in México during 2009 . Math Biosci Eng . 8 ( 1 ): 21 – 48 .
Mena I , et al . 2016 . Origins of the 2009 H1N1 influenza pandemic in swine in Mexico . eLife . 5 : e16777 .
Pourbohloul B , et al . 2009 . Initial human transmission dynamics of the pandemic (H1N1) 2009 virus in North America . Influenza Other Respir Viruses . 3 ( 5 ): 215 – 222 .
Prosper O , et al . 2010 . Modeling control strategies for concurrent epidemics of seasonal and pandemic H1N1 influenza . Math Biosci Eng . 8 ( 1 ): 141 – 170 .
Kraemer MUG , et al . 2020 . Mapping global variation in human mobility . Nature Human Behaviour . 4 ( 8 ): 800 – 810 .
Lemey P , et al . 2021 . Untangling introductions and persistence in COVID-19 resurgence in Europe . Nature . 595 ( 7869 ): 713 – 717 .
Wilson RJ , et al . 2019 . Differentially private SQL with bounded user contribution. arXiv 1909.01917. 10.48550/arXiv.1909.01917 , preprint: not peer reviewed.
Goldstein E , Lipsitch M . 2020 . Temporal rise in the proportion of younger adults and older adolescents among coronavirus disease (COVID-19) cases following the introduction of physical distancing measures, Germany, March to April 2020 . Eurosurveillance . 25 ( 17 ): 2000596 .
Goldstein E , et al . 2018 . On the relative role of different age groups during epidemics associated with respiratory syncytial virus . J Infect Dis . 217 ( 2 ): 238 – 244 .
Goldstein E , Pitzer VE , O’Hagan JJ , Lipsitch M . 2017 . Temporally varying relative risks for infectious diseases: implications for infectious disease control . Epidemiology . 28 ( 1 ): 136 – 144 .
Rosvall M , Bergstrom CT . 2008 . Maps of random walks on complex networks reveal community structure . Proc Natl Acad Sci U S A . 105 ( 4 ): 1118 – 1123 .
Ripley B , et al . 2022 . MASS: support functions and datasets for venables and ripley’s MASS .
Bartoń K . 2022 . MuMIn: multi-model inference . R package version 1.47.1 .
Nakagawa S , Johnson PCD , Schielzeth H . 2017 . The coefficient of determination r2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded . J R Soc Interface . 14 ( 134 ): 20170213 .
Kuznetsova A , Brockhoff PB , Christensen RHB . 2017 . lmerTest package: tests in linear mixed effects models . J Stat Softw . 82 ( 13 ): 1 – 26 .
Zheng L , Chen M , Yang W . 2008 . Random walk in orthogonal space to achieve efficient free-energy simulation of complex systems . Proc Natl Acad Sci U S A . 105 ( 51 ): 20227 – 20232 .
Kraemer MUG , et al . 2020 . The effect of human mobility and control measures on the COVID-19 epidemic in China . Science . 368 ( 6490 ): 493 – 497 .
Monod M , et al . 2021 . Age groups that sustain resurging COVID-19 epidemics in the United States . Science . 371 ( 6536 ): eabe8372 .
Kraemer MUG , et al . 2021 . Spatiotemporal invasion dynamics of SARS-CoV-2 lineage B.1.1.7 emergence . Science . 373 ( 6557 ): 889 – 895 .
Brown TS , et al . 2021 . The impact of mobility network properties on predicted epidemic dynamics in Dhaka and Bangkok . Epidemics . 35 : 100441 .
Schlosser F , et al . 2020 . COVID-19 lockdown induces disease-mitigating structural changes in mobility networks . Proc Natl Acad Sci U S A . 117 ( 52 ): 32883 – 32890 .
Castelán-Sánchez HG , et al . 2023 . Comparing the evolutionary dynamics of predominant SARS-CoV-2 virus lineages co-circulating in Mexico . eLife . 12 : e82069 .
Gutierrez B , et al . 2022 . Emergence and widespread circulation of a recombinant SARS-CoV-2 lineage in North America . Cell Host & Microbe . 30 ( 8 ): 1112 – 1123.e3 .
Hill V , Ruis C , Bajaj S , Pybus OG , Kraemer MUG . 2021 . Progress and challenges in virus genomic epidemiology . Trends Parasitol . 37 ( 12 ): 1038 – 1049 .
Kogan NE , et al . 2021 . An early warning approach to monitor covid-19 activity with multiple digital traces in near real time . Sci Adv . 7 ( 10 ): eabd6989 .
Nouvellet P , et al . 2021 . Reduction in mobility and COVID-19 transmission . Nat Commun . 12 ( 1 ): 1090 .
Fontanelli O , et al . 2023 . Intermunicipal travel networks of Mexico during the COVID-19 pandemic. Sci Rep . 13(1):8566.
Klein B , et al . 2024 . Characterizing collective physical distancing in the U.S. during the first nine months of the COVID-19 pandemic . PLOS Digital Health . 3 ( 2 ): e0000430 .
Mas J-F , Pérez-Vega A . 2021 . Spatiotemporal patterns of the COVID-19 epidemic in Mexico at the municipality level . PeerJ . 9 : e12685 .
van Panhuis WG , et al . 2015 . Region-wide synchrony and traveling waves of dengue across eight countries in Southeast Asia . Proc Natl Acad Sci U S A . 112 ( 42 ): 13069 – 13074 .
Chang S , Vrabac D , Leskovec J , Ugander J . 2023 . Estimating geographic spillover effects of COVID-19 policies from large-scale mobility networks . Proc AAAI Conf Artif Intell . 37 ( 12 ): 14161 – 14169 .
Keeling MJ , Bjørnstad ON , Grenfell BT . 2004 . Metapopulation dynamics of infectious diseases. In: Hanski I, Gaggiotti OE, editors. Ecology, genetics and evolution of metapopulations . London: Elsevier. p. 415–445 .
Metcalf CJE , Munayco CV , Chowell G , Grenfell BT , Bjørnstad ON . 2011 . Rubella metapopulation dynamics and importance of spatial coupling to the risk of congenital rubella syndrome in Peru . J R Soc Interface . 8 ( 56 ): 369 – 376 .
Nicolau PG , Ims RA , Sørbye SH , Yoccoz NG . 2022 . Seasonality, density dependence, and spatial population synchrony . Proc Natl Acad Sci U S A . 119 ( 51 ): e2210144119 .
Valdano E , Okano JT , Colizza V , Mitonga HK , Blower S . 2022 . Use of mobile phone data in HIV epidemic control . Lancet HIV . 9 ( 12 ): e820 – e821 .
Newman M . 2006 . Modularity and community structure in networks . Proc Natl Acad Sci U S A . 103 ( 23 ): 8577 – 8582 .
Pei S , Yamana TK , Kandula S , Galanti M , Shaman J . 2021 . Burden and characteristics of COVID-19 in the United States during 2020 . Nature . 598 ( 7880 ): 338 – 341 .
Clauset A , Moore C , Newman M . 2008 . Hierarchical structure and the prediction of missing links in networks . Nature . 453 ( 7191 ): 98 – 101 .
Galindo-Pérez MC , et al . 2022 . Territorial strategy of medical units for addressing the first wave of the COVID-19 pandemic in the metropolitan area of Mexico city: analysis of mobility, accessibility and marginalization . Int J Environ Res Public Health . 19 ( 2 ): 665 .
Kishore N , et al . 2022 . Evaluating the reliability of mobility metrics from aggregated mobile phone data as proxies for SARS-CoV-2 transmission in the USA: a population-based study . The Lancet Digital Health . 4 ( 1 ): e27 – e36 .
Joint Research Centre (European Commission) , Iacus S , et al . 2020 . Mapping mobility functional areas (MFA) using mobile positioning data to inform COVID-19 policies: a European regional analysis . Luxembourg : Publications Office of the European Union .
Google Preview
de Anda-Jáuregui G , García-García L , Hernández-Lemus E . 2022 . Modular reactivation of Mexico city after COVID-19 lockdown . BMC Public Health . 22 ( 1 ): 961 .
De Groeve T , et al . 2020 . Scenarios and tools for locally targeted covid-19 non pharmaceutical intervention measures . Technical Report KJ-NA-30523-EN-N (online). Luxembourg: Office of the European Union .
Ruktanonchai NW , et al . 2020 . Assessing the impact of coordinated COVID-19 exit strategies across Europe . Science . 369 ( 6510 ): 1465 – 1470 .
Althouse BM , et al . 2023 . The unintended consequences of inconsistent closure policies and mobility restrictions during epidemics . BMC Global Public Health . 1 ( 1 ): 28 .
Agren D . 2020 . Mexico flying blind as lack of COVID-19 testing mystifies experts. The Guardian .
GOB.MX. Exceso de mortalidad en méxico . https://coronavirus.gob.mx/exceso-de-mortalidad-en-mexico/
Thomson Reuters Foundation . Mexico’s indigenous towns impose their own coronavirus lockdowns .
Richterich P . 2020 . Severe underestimation of covid-19 case numbers: effect of epidemic growth rate and test restrictions. medRxiv 2020.04.13.20064220. 10.1101/2020.04.13.20064220 , preprint: not peer reviewed.
Tsang TK , et al . 2020 . Effect of changing case definitions for covid-19 on the epidemic curve and transmission parameters in mainland China: a modelling study . Lancet Public Health . 5 ( 5 ): e289 – e296 .
Ben-Gal I , Weinstock S , Singer G , Bambos N . 2019 . Clustering users by their mobility behavioral patterns . ACM Trans Knowl Discov Data (TKDD) . 13 ( 4 ): 1 – 28 .
Benabdelkrim M , Robardet C , Savinien J . 2021 . Leveraging semantic for community mining in multilayer networks. In: Canadian Conference on AI , Vancouver, Canada. Canadian Artificial Intelligence Association.
Buchel O , Ninkov A , Cathel D , Bar-Yam Y , Hedayatifar L . 2021 . Strategizing covid-19 lockdowns using mobility patterns . R Soc Open Sci . 8 ( 12 ): 210865 .
Smiljanić J , et al . 2023 . Community detection with the map equation and infomap: theory and applications . arXiv arXiv:2311.04036. 10.48550/arXiv.2311.04036 , preprint: not peer reviewed.
Zhang W , et al . 2022 . Structural changes in intercity mobility networks of China during the covid-19 outbreak: a weighted stochastic block modeling analysis . Comput Environ Urban Syst . 96 : 101846 .
Consejo de Salubridad General. https://www.gob.mx/csg
Oliu-Barton M , et al . 2022 . The effect of COVID certificates on vaccine uptake, health outcomes, and the economy . Nat Commun . 13 ( 1 ): 3942 .
López-Gatell y gobernadores chocan por responsabilidades ante COVID-19, 2020 . https://politica.expansion.mx/mexico/2020/07/30/lopez-gatell-y-gobernadores-chocan-por-responsabilidades-ante-covid-19
McCrone JT , et al . 2022 . Context-specific emergence and growth of the SARS-CoV-2 delta variant . Nature . 610 ( 7930 ): 154 – 160 .
Klein B , Hoel E . 2020 . The emergence of informative higher scales in complex networks . Complexity . 2020 : 8932526 .
Peixoto TP . 2019 . Bayesian stochastic blockmodeling. In: Doreian P, Batagelj V, Ferligoj A, editors. Advances in network clustering and blockmodeling . Hoboken (NJ): John Wiley & Sons. p. 289–332 .
Author notes
Supplementary data.
| Month: | Total Views: |
|---|---|
| July 2024 | 6 |
| August 2024 | 111 |
| September 2024 | 20 |
Email alerts
Citing articles via.
- Contact PNAS Nexus
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2752-6542
- Copyright © 2024 National Academy of Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
COMMENTS
This article explores the possible adverse effects of COVID-19 mRNA vaccines, based on the hypothesis that the spike protein may cause tissue damage and inflammation.
The Moderna COVID-19 vaccine is recommended for persons 18 years of age and older in the U.S. population under the FDA's Biologics License Application. *Overall vaccine efficacy was calculated at ≥14 days after second dose of vaccine among persons without evidence of prior SARS-CoV-2 infection.
Trougakos and colleagues recently discussed the role of coronavirus disease 2019 (COVID-19) mRNA vaccine-induced spike (S) protein in adverse effects following vaccination [1]. We would like hereafter to answer one of their outstanding questions requiring response to improve efficacy and safety of COVID-19 vaccines, and to propose an additional ...
The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety. Download a PDF of the Research Summary.
Vaccines are needed to prevent coronavirus disease 2019 (Covid-19) and to protect persons who are at high risk for complications. The mRNA-1273 vaccine is a lipid nanoparticle-encapsulated mRNA ...
To increase statistical power and meet vaccine success criteria, we propose to evaluate the efficacy of coronavirus disease 2019 (COVID-19) vaccines by using the dual or triple primary end points of severe acute respiratory syndrome coronavirus 2 infection, symptomatic COVID-19, and severe COVID-19. There is an urgent need to develop effective ...
Vaccination is a major tool for mitigating the coronavirus disease 2019 (COVID-19) pandemic, and mRNA vaccines are central to the ongoing vaccination campaign that is undoubtedly saving thousands of lives. However, adverse effects (AEs) following vaccination have been noted which may relate to a proinflammatory action of the lipid nanoparticles ...
At interim analysis in a phase 3, observer-blinded, placebo-controlled clinical trial, the mRNA-1273 vaccine showed 94.1% efficacy in preventing coronavirus disease 2019 (Covid-19). After emergency...
In a dose-escalation study of the COVID-19 RNA vaccine BNT162b1 in 45 healthy adults, RBD-binding IgG concentrations and SARS-CoV-2 neutralizing titres in sera increased with dose level and after ...
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Vaccination is a major tool for mitigating the coronavirus disease 2019 (COVID-19) pandemic, and mRNA vaccines are central to the ongoing vaccination campaign that is undoubtedly saving thousands of lives. However, adverse effects (AEs) following vaccination have been noted which may relate to a pro …
This cohort study compares the risk of potential adverse events between mRNA vaccines mRNA-1273 and BNT162b2 for COVID-19 among older US adults.
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute ...
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
Information about all of the updated COVID-19 vaccines (2024-2025 formula) available in the United States, including fact sheets, package inserts and supporting documents, can be found here.
The trial end points were vaccine efficacy against laboratory-confirmed Covid-19 and safety, which were both evaluated through 6 months after vaccination. Download a PDF of the Research Summary.
After reviewing the available literature, we are particularly concerned that certain COVID-19 vaccines may generate a pro-tumorigenic milieu (i.e., a specific environment that could lead to neoplastic transformation) that predisposes some (stable) oncologic patients and survivors to cancer progression, recurrence, and/or metastasis.
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement ...
Vaccine hesitancy was a major challenge during the COVID-19 pandemic. A common but sometimes ineffective intervention to reduce vaccine hesitancy involves providing information on vaccine ...
Health Canada authorized Moderna's updated COVID-19 vaccine on Tuesday to roll out in fall immunization campaigns. The federal vaccine portal lists approval of Moderna's product. Provinces and ...
Doses of Moderna's updated COVID-19 vaccine are expected to begin arriving in Canada 'within days,' a spokesperson for the Public Health Agency of Canada says, although availability will depend on ...
Moderna shares surged Tuesday after Canadian regulators approved the biotech firm's updated COVID-19 vaccine. Moderna's Spikevax KP.2 targets the KP.2 variant of the virus.
The Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein in ...
The challenging part is a need for vigorous testing for immunogenicity, safety, efficacy, and level of protection conferred in the hosts for the vaccines. As the world responds to the COVID-19 pandemic, we face the challenge of an overabundance of information related to the virus.
However, with COVID-19 stubbornly sticking around, investors should focus on an obscure, up-and-coming COVID-19 vaccine maker called Novavax. Novavax Sanofi Deal Offers Distribution and Funding
Unlike the flu and Covid-19 vaccines, the RSV vaccine is one-time dose. Older adults who received an RSV vaccine last year when they first became available do not need to get another one this year.
With the recent wave of COVID-19 cases this summer, the Centers for Disease Control and Prevention (CDC) recommends that everyone 6 months and older receive the updated 2024-2025 COVID-19 vaccine. This is to help protect against potentially serious outcomes this fall and winter, regardless of whether you've been vaccinated before.
Understanding and minimizing coronavirus disease 2019 (COVID‐19) vaccine hesitancy is critical to population health and minimizing health inequities, which continue to be brought into stark relief by the pandemic. We investigate questions regarding ...
Outbreaks of infectious diseases, including COVID-19 across different localities are linked via human mobility. Using aggregated human mobility data at the municipality level in Mexico, we find that scales of human mixing and predictability of COVID-19 growth rates shift during the pandemic which improves the ability to target spatial interventions.