An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Biosensors: Classifications, medical applications, and future prospective
Affiliations.
- 1 Faculty of Applied Medical Sciences, Department of Medical Laboratory Technology, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
- 2 Special Infectious Agent Unit, King Fahd Medical Research Centre, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
- PMID: 29023994
- DOI: 10.1002/bab.1621
Biosensors are devices that combine a biological material with a suitable platform for detection of pathogenic organisms, carcinogenic, mutagenic, and/or toxic chemicals or for reporting a biological effect. In recent years, an enormous number of different types of biosensors have been constructed and developed for several medical applications. The reason for that was primarily due to the numerous advantages and applications that can be offered by biosensors. This review article has been started with demonstrating the power of biosensor technologies versus analytical and conventional techniques. Subsequently, more emphasis has been added on the classification and the role of biosensors in several medical applications such as detection and monitoring of carcinogenic and mutagenic chemicals, reporting of endocrine disrupting compounds, and detection of pathogenic organisms. The most common reporter genes used in biosensors engineering and construction have also been summarized. Prospective strategies and recommendations for the future construction of biosensors have been highlighted.
Keywords: Bioassays; Biofilms; Bioluminescence; Bioreporters; Biosensors; Carcinogenicity; DNA Aptamer; EDCs; Genotoxicity; Immunosensors; Microfluidic; Nanobiotechnology; Pathogenic Microorganisms; Reporter Genes; Reporter Phage; SOS-lux; organ-on-a-chip.
© 2017 International Union of Biochemistry and Molecular Biology, Inc.
PubMed Disclaimer
Similar articles
- The potential applications of SOS-lux biosensors for rapid screening of mutagenic chemicals. Alhadrami HA, Paton GI. Alhadrami HA, et al. FEMS Microbiol Lett. 2013 Jul;344(1):69-76. doi: 10.1111/1574-6968.12156. Epub 2013 May 1. FEMS Microbiol Lett. 2013. PMID: 23581454
- Biosensors for environmental monitoring of endocrine disruptors: a review article. Rodriguez-Mozaz S, Marco MP, Lopez de Alda MJ, Barceló D. Rodriguez-Mozaz S, et al. Anal Bioanal Chem. 2004 Feb;378(3):588-98. doi: 10.1007/s00216-003-2385-0. Epub 2003 Nov 29. Anal Bioanal Chem. 2004. PMID: 14647938 Review.
- Enhanced detection of endocrine disrupting chemicals in on-chip microfluidic biosensors using aptamer-mediated bridging flocculation and upconversion luminescence. Wu J, Wei W, Ahmad W, Li S, Ouyang Q, Chen Q. Wu J, et al. J Hazard Mater. 2023 Sep 15;458:132025. doi: 10.1016/j.jhazmat.2023.132025. Epub 2023 Jul 11. J Hazard Mater. 2023. PMID: 37453351
- Hazard identification of endocrine-disrupting carcinogens (EDCs) in relation to cancers in humans. Sharma N, Kumar V, S V, Umesh M, Sharma P, Thazeem B, Kaur K, Thomas J, Pasrija R, Utreja D. Sharma N, et al. Environ Toxicol Pharmacol. 2024 Aug;109:104480. doi: 10.1016/j.etap.2024.104480. Epub 2024 May 31. Environ Toxicol Pharmacol. 2024. PMID: 38825092 Review.
- Prêt-à-porter nanoYESα and nanoYESβ bioluminescent cell biosensors for ultrarapid and sensitive screening of endocrine-disrupting chemicals. Lopreside A, Calabretta MM, Montali L, Ferri M, Tassoni A, Branchini BR, Southworth T, D'Elia M, Roda A, Michelini E. Lopreside A, et al. Anal Bioanal Chem. 2019 Jul;411(19):4937-4949. doi: 10.1007/s00216-019-01805-2. Epub 2019 Apr 10. Anal Bioanal Chem. 2019. PMID: 30972468
- Genotoxicity and mutagenicity assessment of electronic cigarette liquids. Al-Otaibi HM, Baqasi AM, Alhadrami HA. Al-Otaibi HM, et al. Ann Thorac Med. 2024 Jul-Sep;19(3):222-227. doi: 10.4103/atm.atm_59_24. Epub 2024 Jul 4. Ann Thorac Med. 2024. PMID: 39144536 Free PMC article.
- Integrating optical and electrical sensing with machine learning for advanced particle characterization. Kokabi M, Tayyab M, Rather GM, Pournadali Khamseh A, Cheng D, DeMauro EP, Javanmard M. Kokabi M, et al. Biomed Microdevices. 2024 May 23;26(2):25. doi: 10.1007/s10544-024-00707-0. Biomed Microdevices. 2024. PMID: 38780704 Free PMC article.
- Structural basis of transcription factor YhaJ for DNT detection. Kim M, Kang R, Jeon TJ, Ryu SE. Kim M, et al. iScience. 2023 Sep 21;26(10):107984. doi: 10.1016/j.isci.2023.107984. eCollection 2023 Oct 20. iScience. 2023. PMID: 37822509 Free PMC article.
- Nanosensors in the detection of antihypertension drugs, a golden step for medication adherence monitoring. Mobed A, Gholami S, Tahavvori A, Ghazi F, Masoumi Z, Alipourfard I, Naderian R, Mohammadzadeh M. Mobed A, et al. Heliyon. 2023 Aug 27;9(9):e19467. doi: 10.1016/j.heliyon.2023.e19467. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810167 Free PMC article. Review.
- Biosensors, Recent Advances in Determination of BDNF and NfL. Mobed A, Charsouei S, Yazdani Y, Gargari MK, Ahmadalipour A, Sadremousavi SR, Farrahizadeh M, Shahbazi A, Haghani M. Mobed A, et al. Cell Mol Neurobiol. 2023 Nov;43(8):3801-3814. doi: 10.1007/s10571-023-01401-0. Epub 2023 Aug 21. Cell Mol Neurobiol. 2023. PMID: 37605014 Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources, other literature sources.
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Next Article
Introduction
General overview of biosensors, classification of biosensors based on the transduction methods, characteristics of biosensors, limit of detection, fields of biosensor applications, machine learning applications in biosensing, conclusions, acknowledgment, author contribution statement, conflict of interest, data availability statement, a review of biosensors and their applications.
These authors contributed equally.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Permissions
- Search Site
Katey, B., Voiculescu, I., Penkova, A. N., and Untaroiu, A.A Review of Biosensors and Their Applications ASME Open J. Engineering ASME. January 2023 2 020201 doi: https://doi.org/10.1115/1.4063500
Download citation file:
- Ris (Zotero)
- Reference Manager
This paper reviews sensors with nano- and microscale dimensions used for diverse biological applications. A biosensor converts biological responses into electrical signals. In recent years, there have been significant advancements in the design and development of biosensors that generated a large spectrum of biosensor applications including healthcare, disease diagnosis, drug delivery, environmental monitoring, and water and food quality monitoring. There has been significant work to enhance the performance of biosensors by improving sensitivity, reproducibility, and sensor response time. However, a key challenge of these technologies is their ability to efficiently capture and transform biological signals into electric, optic, gravimetric, electrochemical, or acoustic signals. This review summarizes the working principle of a variety of biosensors in terms of their classification, design considerations, and diverse applications. Other lines of research highlighted in this paper are focused on the miniaturization of biosensing devices with micro and nano-fabrication technologies, and the use of nanomaterials in biosensing. Recently wearable sensors have had important applications such as monitoring patients with chronic conditions in home and community settings. This review paper mentions applications of wearable technology. Machine learning is shown to help discover new knowledge in the field of medical applications. We also review artificial intelligence (AI) and machine learning (ML)-based applications.
This extensive review presents different types of biosensors with nano and microscale dimensions used for biological applications. A biosensor is an analytical device, used for the detection of an analyte, that combines a sensitive biological component with a detector element (transducer) [ 1 – 3 ]. The sensitive biological element could be a bioreceptor, a biomimetic component, or a biologically derived material that interacts or binds with the analyte under study. A wide range of biological materials are used for the biosensors such as aptamers, tissue, microorganisms, organelles, mammalian cells, bacteria, enzymes, antibodies, and nuclei acids [ 3 – 15 ]. The transducer, which transforms the biological signal into a physio-chemical signal, has different detection options: electrochemiluminescence [ 16 – 19 ], optical [ 20 , 21 ], electrochemical [ 22 , 23 ], fluorescence [ 24 , 25 ], piezoelectric [ 26 ], etc., resulting from the interaction of the analyte with the sensitive biological element. Figure 1 provides an illustration of the principle of biosensors.

Illustration of principal components of a biological sensor device: (1) bioreceptor–detector layer of immobilized biomaterial; (2) physicochemical transducer, (3) signal-conditioning and recording the signal for human interpretation
The use of biosensors was pioneered by Clark and Lyons [ 27 ]. In the early 1960s, they developed an electrode that could measure the oxygen concentration in blood. In this research, the electrode was introduced in perfusion blood during an open-heart surgery procedure. Biosensors have the capability to respond to specific biological signals and process them into measurable quantities for human comprehension. Recently, novel biosensors that can be implantable or wearable were developed [ 28 ]. In general, a biosensor consists of three main components. These include the biological sensing component for recognition of the biological signal, the transducer for converting the biological signal into an electric signal, and the signal processing system, which amplifies and displays the output in a desired format for human understanding [ 29 ]. The applications of biosensors are diverse; they have proven their usefulness in disease diagnosis in human healthcare delivery, agriculture, homeland security, food security, environmental, and industrial monitoring, as well as bioprocessing [ 28 ].
Biological sensors can monitor in real time, the vital signs of individual patients for a long period of time, and provide them with personalized health solutions. For achieving these goals, the biological sensors could be skin-integrated in wearable systems, or implantable medical devices. These biosensors are important to monitor different biophysical parameters of the patients such as; blood pressure, blood glucose level, heart electrical signals, pulse rate, and respiration rate [ 30 ]. The engineers and scientists have consistently collaborated to improve the biosensors' performance with emphasis on biocompatibility, specificity, reliability, durability, and consistency, as well as sensitivity for diagnosis, monitoring, and treatment of several health conditions.
In addition to real-time monitoring of patients and decisions connected to patient health complications, there is also the need for biosensors to simultaneously detect multiple analytes and/or respond to more than one stimulus, within biological fluids, within or outside the human body [ 29 ]. Unlike many other sensors used in other fields of application, biosensors (both implantable and non-implantable) are designed with unique features such as biocompatibility, biodegradability, and/or the ability to be bioresorbable, and miniaturization due to the biological environment in which they function. For instance, a skin-integrated device must be flexible, stretchable, lightweight, ultra-thin, and reliable. These devices can conform to the geometry and/or shape of the mounting surface. Furthermore, any regular movement or modification of the skin must be allowed without causing any discomfort to the patient [ 28 ].
This review paper is focused on several aspects of biosensing such as a general overview of biosensors, classification of biosensors based on the transduction methods, characteristics of biosensors, biosensor design considerations, biosensor applications, advancement in biosensors due the nanomaterials discovery, wearable biosensors, and machine learning application in biosensing.
Biosensors have the capability of responding to the presence of a specific biological analyte, and the ability to quantify the analyte, subsequently translating it into a signal that is human-readable [ 3 , 31 – 33 ]. The sensory element is biochemical in nature, and the biosensor operation is based on reactions with the analyte of interest. A biosensor consists of three main components: (1) bioreceptor–detector layer of immobilized biomaterial; (2) physio-chemical transducer, which transforms the biological response into a measured electric signal; (3) electronic system for amplifying and recording the signal for human interpretation [ 34 , 35 ]. The bioreceptor is simply a biological molecule that comes into direct contact with the analyte of interest. Generally, it is immobilized on the active surface of the transducer. The responsibility of the bioreceptor is to detect the presence of the analyte and subsequently bind it. This results in physiological changes, which then also modify the physicochemical properties of the transducer in the vicinity of the biological receptor. This phenomenon leads to changes in the physical properties of the transducer, which are translated into an electrical signal for human understanding [ 36 ]. These main components of the biosensor are illustrated in Fig. 1 .
According to Inshyna et al. [ 37 ], biosensors can be classified based on either the type of transducer or type of bioreceptor used for their construction. According to this classification, we have electrochemical, optical, calorimetric, piezoelectric, acoustic, and electronic biosensors. Furthermore, based on the type of bioreceptors, biosensors can also be grouped as follows: enzymatic, cellular, tissular, DNA sensors, immunosensors, and aptamer sensors [ 34 , 38 , 39 ].
Electrochemical Transducers.
Electrochemical biosensors exhibit high sensitivity, selectivity, and the capability to detect. Electrochemical biosensors are based on classical electrochemistry, with the electrodes introduced in an electrolyte. The electrochemical reaction occurs at the working electrode surface between bioreceptor and analyte producing detectable electrochemical signals in terms of voltage, current, impedance, and capacitance. The devices used for electrochemical measurements are relatively simple, easily miniaturized, require low power, and are sensitive for biological applications.
In experimental electrochemistry, the three-electrode cell is one of the most common configurations used to study electrochemical reactions. It consists of a counter electrode, working electrode, and reference electrode, as illustrated in Fig. 2 . The electrode where oxidation/reduction takes place (where the potential is controlled) is the working electrode. The reference electrode has a stable potential; no current flows through it. The potential of the working electrode is measured relative to it. The potentiostat is used to supply a constant potential to the working electrode, regardless of the chemical changes taking place on the working electrode at that time. Due to the advancement of microelectromechanical systems’ (MEMS) microfabrication, the classic electrochemical cells could be miniaturized at microscale [ 40 ]. Figure 3 is an illustration of three electrodes miniaturized electrochemical cells that can be used for the detection of biomarkers in blood or biological fluids.

Electrochemical measurements with a three-electrode potentiostat
![research papers on biosensors Schematic of microfluidic system for testing blood biomarkers using microscale electrochemical electrodes. The working electrode could be planar or covered with tall pillars. Reproduced with permission from Pandikumar and Rameshkumar [41].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f003.png?Expires=1726586656&Signature=DTRjqeu~uhpgmbjtEGcUWt6Q46zlR4yDZ377WIXg0sGVhkuo4WCq5EC0Gu-MVnk3CQ7~4rhiXkUFlsjC6G-jWFdJbZ02oIenjhFH6g3cFYAsSWi8wEzyR3XnRq~KXrMPoeBIU5DWVZFiERC0EKfQRYMtM7M7tbCscdKJuL-h4eCU5jX~EbEu1BMEwPQN2ELcot176dyJX5Oqf4oWW0-hNYnZBfP-JxBAbecEKCdVenjmpGbmKPShzf33jCnvJVq7Kpex4aSBoolt8TYDkd6dLTBlvcvQ67Utdlc7JSRlxP0qarfVqMweUpzXXveYPvWkeaXlPbS4MFWpBJl5q6uc2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic of microfluidic system for testing blood biomarkers using microscale electrochemical electrodes. The working electrode could be planar or covered with tall pillars. Reproduced with permission from Pandikumar and Rameshkumar [ 41 ].
Based on the transduction principle, electrochemical biosensors are categorized as (a) voltametric, (b) amperometric, (c) impedimetric, (d) conductometric, and (e) potentiometric [ 42 , 43 ]. These sensors convert the information associated with electrochemical reactions (the reaction between an electrode and analyte) into an applicable qualitative or quantitative signal [ 41 ]. In this review, we will discuss voltametric and impedimetric biosensors because there are many applications for these sensors.
The working electrode could be a simple planar electrode or contain an array of micropillars fabricated on the planar working electrode. The micropillar electrode has a larger active area for detection to enhance the sensitivity of detection (Fig. 4 ).
![research papers on biosensors Scanning electron microscopic images of the working electrodes. (a)–(c) Micropillar electrodes of diameter (a) Ø30 µm, (b) Ø20 µm, and (c) Ø10 µm. The scale bars are 100 µm. (d)–(g) The surface of the flat electrode modified with gold black at the current density of −30 µA/mm2 (d), −60 µA/mm2 (e), −120 µA/mm2 (f), and without the modification (g). The scale bars are 2.5 µm. (h) and (i) The surface of a micropillar of the Ø30 µm electrode modified with gold black at the current density of −60 µA/mm2 (h) and without the modification (i). The scale bars are 10 µm. Reproduced with permission from Numthuam et al. [40].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f004.png?Expires=1726586656&Signature=RqJnG67hqhcuO5E1euhcYR0ZtKSzYzq-o1GuAvuxgd9RD8b1uDekqLGGZn23RqI2JTpiXwuSk-s9Oy6k8JXpO4egU2ELrVyV7~a-5v~8nXULJuP5Cx0zQU3rZX0lDIBQqzrkQ7XfBTrH6gtQASz~QAr7rIXSrnZ8EdBKST~oc8KHBac6XYL3fhB0a488q8yIwiOkR-m7018qsQVJvQ3BjZ-Z7VG7pypixbN0shIFuZETbgg1jL1InFtvwPdxcqk6qXjw3epI-lc8Q1Hq8REuG3qu24peKUHjPH30EA5GrPNPQDlrRQVslZoC4p9dzi9aBfCdBVV3VJvVP3uVtGmpkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Scanning electron microscopic images of the working electrodes. ( a ) – ( c ) Micropillar electrodes of diameter ( a ) Ø30 µ m, ( b ) Ø20 µ m, and ( c ) Ø10 µ m. The scale bars are 100 µ m. ( d ) – ( g ) The surface of the flat electrode modified with gold black at the current density of −30 µ A/mm 2 ( d ), −60 µ A/mm 2 ( e ), −120 µ A/mm 2 ( f ), and without the modification ( g ). The scale bars are 2.5 µ m. ( h ) and ( i ) The surface of a micropillar of the Ø30 µ m electrode modified with gold black at the current density of −60 µ A/mm 2 ( h ) and without the modification ( i ). The scale bars are 10 µ m. Reproduced with permission from Numthuam et al. [ 40 ].
Voltametric and amperometric techniques are characterized by applying a potential to a working electrode versus a reference electrode and measuring the current. The term voltammetry is used for those techniques in which the potential is scanned over a set potential range. The current response is usually a peak or a plateau that is proportional to the concentration of the analyte. In amperometry, changes in the current generated by the electrochemical oxidation or reduction are monitored directly with time, while a constant potential is maintained at the working electrode with respect to a reference electrode. The voltametric/amperometric biosensors generally are used for enzyme-linked immunosorbent assays (ELISA) detection, and there is extensive research in this area [ 40 , 44 ].
Impedimetric biosensors measure changes in conductance and capacitance at the sensor surface as the selective binding of the target occurs. In this way, the impedimetric transducers can detect biological molecules or the behavior of live cells. For applications regarding live cells, the impedimetric transducer is named Electric Cell-substrate Impedance Sensing (ECIS), which is a real-time and label-free detection method to analyze the behavior of cells. The ECIS technique was pioneered by Giaever and Keese [ 45 – 47 ] and has been extensively studied for over two decades due to its simple structure, easy operation, and sensitivity to many cell behaviors and properties [ 48 – 55 ]. The ECIS technique can measure cell attachment, proliferation, migration, invasion, and cell viability because the impedance measurements are directly responding to cell attachment, growth, and proliferation. When cells attach to the electrodes, cell attachments result in additional impedance to the circuit. Their insulating properties can be detected. The impedance values gradually increase until monolayer formation and then reach equilibrium when the cells are confluent and stable. Apoptotic cells lose the dielectric properties and tend to detach from the sensing electrodes. When the cells detach or lose the dielectric properties, the measured membrane impedance will decrease. As cells grow and cover the electrodes, information about the morphology of the cells and nature of the cell attachment can be extracted from the measured impedance [ 52 – 55 ].
The name “impedance spectroscopy” is derived from the fact that the impedance is generally determined at different frequencies rather than just one. The impedance spectroscopy measurements are generally performed using a small Alternative Current (AC) electric field over a wide frequency range (100–100 kHz). Thus, an impedance spectrum is obtained that allows the characterization of cell size, membrane resistance and capacitance, and cytoplasm conductivity as a function of frequency.
Determining the experimental values, for the capacitance and resistance of the cell membrane, enables cell electrophysiological information collection [ 52 – 55 ]. As an example, blocking the ion channels enables the cell membrane to act more capacitively. The capacitance and resistance characterizing a live cell are significantly different from those of a dead cell. While healthy cells adhere more securely to surfaces, as compared to unhealthy or dead cells, this results in a stronger capacitive coupling between the cells and underlying electrodes. When the cells die, the value of impedance reduces, due to the change in the membrane capacitance and resistance.
The ECIS technique has become convenient for various applications, such as: (1) monitoring cell migration and wound repair [ 56 ], (2) monitoring cell proliferation [ 57 ], (3) real-time continuous trans-epithelial electrical resistance (TEER) measurements [ 58 ], (4) monitoring the signal transduction pathways activated by G-protein-coupled receptors (GPCR) [ 59 ], (5) studying cell differentiation events [ 60 ], (6) assessing the cytotoxicity of a variety of toxicants [ 61 ], and (7) monitoring the cell attachment and spreading [ 62 ].
Recently, a review of the ECIS technique used for applications of two-dimensional cell culture was published in the literature [ 63 ]. For instance, ECIS has important applications in the study of cancer metastasis [ 49 , 53 , 64 ]. Mathematical models are used to simulate the impedance of cell monolayer and the influence of electrode dimensions on the detection sensitivity [ 54 , 55 ]. The cell viability was also tested using the ECIS technique, as reported in Ref. [ 65 ].
In the past decade, investigation into stretchable electronics has been widely conducted. Dr. John A. Rogers’ group at the University of Illinois, Urbana-Champaign invented the stretchable electronics concept. According to Refs. [ 66 , 67 ], advanced mechanics and materials research have made possible the design of flexible electronics, such as integrated circuits with properties and functionalities comparable to acoustic sensors of the traditional silicon wafer-based ones. The advantages associated with stretchable electronic technology are its ability to conform to irregular surfaces and shapes without any distortion in their functions or deformation. Other advantages include the possibility of micro and nanoscale electronic designs that otherwise are not feasible with the conventional silicon wafer-based type. The advent of stretchable electronics has paved the way for the design of electronic eyeball cameras and deformable light-emitting displays. It is safe to say that soon, low-power sensors can be designed using flexible electronic technology together with piezoelectric materials. Figure 5 shows examples of stretchable electronics.

Examples of stretchable electronics. ( a ) Stretchable silicon circuit in a wavy geometry, compressed in its center by a glass capillary tube (main) and wavy logic gate built with two transistors (top right inset). ( b ) Stretchable silicon circuit with a mesh design, wrapped onto a model of a fingertip, shown at low (left), moderate (center) and high (right) magnification. The red (left) and blue (center) boxes indicate the regions of magnified views in the center and right, respectively. The image on the right was collected with an automated camera system that combines images at different focal depths to achieve a large depth of field. ( c ) Array of organic transistors interconnected by elastic conductors on a sheet of PDMS in a stretched (left) and curvilinear (right) configuration.
A stretchable biosensor with the ECIS technique was also demonstrated [ 68 ]. Authors reported that ECIS electrodes were embedded in a stretchable device and ECIS measurements on mammalian cells exposed to a cyclic strain were performed. The stretchable ECIS sensor was demonstrated to be capable of real-time monitoring the cell proliferation, while applying mechanical stimulation [ 68 ]. Figure 6 shows a stretchable ECIS sensor and details of the components.
![research papers on biosensors Stretchable ECIS sensor. A linear motor was used to cyclically stretch the ECIS sensor. (a) The linear motor with the stretchable ECIS sensor mounted on it was placed inside the incubator; (b) stretchable ECIS sensor. Reproduced with permission from Zhang et al. [68].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f006.png?Expires=1726586656&Signature=glWBvH13-6MyABxBiZFkUJbCjan17WqRABXSuxj65OndsbXpavrXazOYiyR70tCZrlpIQWhrgo14QkLp-EXRfFg~C60kwnOU5YFEHukM8sUcyw~crvU6vymWPd-TQSfV5c4wHfRePlqHUO5m0C9f6JWdxCIO1Zz4RMV023ZfJ-5EcMSJJ2E1~yWSaZ~cJn4JsyQBNU~bqQ5Gzbee7LrktGjQkby2P2HdMJrAKypza-2DfHwe82EKWwBiGJldLnhYAhJX4zVVoDehEzbnAH5ag6lGC1BwmGPcSvvyDzG~NMFesNT8HJH0RJnCgDILT3ttNcJnWjr-GWpT-ZTjuoyiBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Stretchable ECIS sensor. A linear motor was used to cyclically stretch the ECIS sensor. ( a ) The linear motor with the stretchable ECIS sensor mounted on it was placed inside the incubator; ( b ) stretchable ECIS sensor. Reproduced with permission from Zhang et al. [ 68 ].
Electrochemical biosensors have an important role in many clinical, environmental, industrial, pharmaceutical, defense, and security applications due to their superior sensitivity and selectivity. Recent developments in nanotechnology and material science, as well as being able to custom engineer the biorecognition component, will further push the development of useful and reliable electrochemical biosensor devices. The sometimes-limited shelf life and stability of the biorecognition component, as well as nonspecific binding, continue to be the biggest limitations for these types of biosensors. However, many strategies have helped to overcome or minimize these problems.
Piezoelectric-Based Biosensors.
The Quartz crystal microbalance (QCM) is a classic example of a piezoelectric biosensor. A standard QCM is based on a thin quartz plate with two aligned circular electrodes fabricated at the top and bottom surfaces of the piezoelectric substrate (Fig. 7 ). The QCM operates using thickness-shear mode. When an AC is applied between the top and bottom electrodes, the top and bottom surfaces of the piezo plate extend in the opposite direction parallel to the plate surface, which generate a thickness shear mode that propagates through the quartz substrate. Because of the high accuracy and stability of QCM measurements [ 69 – 75 ], this technique has become helpful for scientists, especially for viscosity and density sensing purposes. The drawback of the QCM measurements is the damping that occurs when the biological liquid is added to the top electrode, further decreasing the QCM sensibility. Generally, the QCM resonant frequency variations are monitored in real time and give information about the process that occurs at the piezoelectric crystal surface level.
![research papers on biosensors Fabrication of culturing chamber for commercial QCM. (a) Cross-view of the PDMS chambers. Two layers of PDMS were used to obtain PDMS with tall dimensions. (b) Photographic images of the commercial QCM with the fabricated culturing chamber used for the experiments. Reproduced with permission from Lee et al. [69].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f007.png?Expires=1726586656&Signature=gtrtCnsaO-hrMbX69oR2QghQCqv8mIaeIBKjseH5JgJVnfLdTd4kmRUbtRTA~MlyjetoN2mBWoaZYAKBhhUTQTvi9QlnhTU-Fu2uLOOp0Hh5RUFELJQRhGi-622vdM6WH0w7lPrgi~90aHNv0cKr52VaOU0Vgr-bV51sbJDsHtsKm27s7yXrdH3YkXeFlED-1m~fc2Nhfp2oHfrXT~nJEsimLtjJ-~lEe8SQ9rwnwQkVQaDY82eILkdjF0TW4k1loLGSaAY6F5CCbsOB671JyT-LpJyOWUIOcjAW-fKD4ce0aR-GbC6eg0bsKEjUQONOosUnvIqvEEn6H4uMHJqIPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Fabrication of culturing chamber for commercial QCM. ( a ) Cross-view of the PDMS chambers. Two layers of PDMS were used to obtain PDMS with tall dimensions. ( b ) Photographic images of the commercial QCM with the fabricated culturing chamber used for the experiments. Reproduced with permission from Lee et al. [ 69 ].
The QCM operates in the resonant frequency range between 1 MHz and 20 MHz, although at higher frequencies the QCM provides good opportunities for enhancing the sensitivity of the detection. To achieve high resonant frequency, QCM biosensors create technological complications during microfabrication, because the piezoelectric substrate must be very thin [ 76 ]. Generally, the QCM detects biological molecules by measuring the changes in resonant frequency that occur when the antigen binds to the antibody receptor [ 77 ].
QCM detection offers stability, reusability, and cost-effectiveness. There are many research papers describing the biological detection with QCM. The QCM is an extremely sensitive mass sensor, capable of measuring mass changes in the nanogram range. This piezoelectric thickness-shear-mode resonator has been successfully employed for biological sensing applications, due to the minimal damping of the acoustic wave in liquid. The QCM's sensitive surface can be functionalized with different molecules that respond to different target analytes and have been tested with a wide range of biological applications. The adhesion [ 78 , 79 ] and viscoelastic [ 80 – 82 ] properties of mammalian cell monolayers have been successfully characterized and reported using QCM resonators. QCM was previously used for virus detection. The QCM sensor was able to detect 1 nanogram of orchid virus [ 83 , 84 ]. QCM was also used for the detection of avian influenza virus [ 85 ].
An interesting application of the QCM is its combination with ECIS electrodes. Mammalian cells were in vitro cultured on the QCM and ECIS electrodes. This combination of ECIS and QCM devices was used as a water toxicity sensor. The mammalian cells were the sensorial element and their viability after exposure to the toxicant was monitored with both sensors: ECIS and QCM [ 70 , 71 ]. A commercial QCM was used to investigate live cells’ activity in water-based toxic solutions. The QCM used in this research had a resonant frequency of 10 MHz and consisted of an AT-cut quartz crystal with gold electrodes on both sides. This QCM was transformed into a functional biosensor by integrating with polydimethylsiloxane (PDMS) culturing chambers (Fig. 7 ) [ 69 ].
Surface Acoustic Wave Devices.
Surface acoustic wave sensors use piezoelectric materials to generate acoustic waves. Surface acoustic waves (SAW) are elastic waves that travel along the surface of the piezoelectric substrate, typically within 1–2 wavelengths from the surface and are sensitive to any modifications on its path of propagation. Anisotropic substrates like Lithium Niobate, Lithium Tantalate, Quartz, and Langasite are commonly used substrates for SAW generation. Parameters like crystal orientation, SAW velocity, coupling coefficient, permittivity and temperature coefficient of delay (TCD) determine the generation of SAW in these substrates [ 86 ].
SAW devices for liquid applications have attracted much attention due to their wide applications in the biological field [ 87 – 89 ]. Among various SAW devices, the wave-guided shear-horizontal (SH) SAW device, also referred to as the Love wave device, has been studied for liquid applications due to its high mass sensitivity and sensing capability in liquid environments. A Love mode surface acoustic wave device uses a piezoelectric substrate with inter-digital transducers (IDTs) to produce the surface-localized SH waves. A material layer with a slower acoustic velocity is deposited on the piezoelectric substrate to trap the acoustic energy near the surface and prevent energy loss into the bulk material. An essential application of the Love mode SAW is immunosensing specific biomarkers [ 87 , 90 – 93 ]. Other than immunosensors, SAW devices were used as mammalian cell and bacteria-based sensors [ 94 ]. In Ref. [ 95 ], a cell-based Love wave SAW biosensor was developed for real-time marine toxin testing.
A surface-horizontal surface acoustic wave (SH-SAW) device was developed for real-time monitoring of blood viscosity at room temperature. The device was tested with a liquid drop placed on the SAW delay-line path and demonstrated a sensitivity of 3.57 ± 0.3125 kHz shift per centipoise, enabling the potential for high-precision blood viscosity monitoring [ 96 ].
SAW sensors have been used in different applications. These include biosensing, chemical and gas sensing, microfluidics, and mechano-biological applications. For biological applications, SAW-based sensors are used for separating, identifying, and controlling biological targets; for instance, biomolecules, or proteins from bio-species, such as bacteria, fungi, or viruses. For chemical and gas sensing, the targeted chemical or gas molecule is attached to the functionalized surface and positioned between two electrodes. A perturbation of the resonant frequency is generated after the targeted biological material is sensed [ 97 ].
Electronic Biosensors Based on Field-Effect Transistors.
These types of transducers employ the use of ion-sensitive silicon field-effect transistors (FET). The bio-sensitive layer is placed above the surface of an ion-sensitive membrane that forms a part of the gate of the field-effect transistor. Conventionally, FET-based biosensors with receptors (e.g., antibody) immobilized on the gate region above the active channel of the FETs face an intrinsic issue, that is the severe charge screening effect in high ionic strength solutions, such as in serum or blood samples, leading to low sensitivity for direct detection of protein in the physiological environment. Several research groups have reported that conventional FET-based biosensors can effectively detect proteins in a physiological salt environment, using AC signals in drain-source voltage (Vds), in conjunction with a reference electrode, in a relatively high frequency [ 98 – 101 ]. An extensive review of the FET presents diverse and interesting applications of this type of biosensor [ 102 ].
The FET transistors were demonstrated to feasibly measure Aβ fibrils in human serum with concentrations ranging from 100 pM to 10 μ M [ 103 ]. C-Reactive protein (CRP) was sensed from 1 fM to 100 nM, demonstrating the high sensitivity of this type of biosensor [ 104 ]. Nanowires (NW) and nanoribbon (NR) were used in the fabrication of the FET biosensor. The detection in the subthreshold regime of NW FET sensor has the merit of not only improving the conductance response and signal-to-noise ratio but also better detection limit. The research showed that the detection limit of the NW FET device was improved from ∼0.75 pM in the linear regime to ∼1.5 fM in the subthreshold regime [ 105 ]. In comparison with the nanowire structure, the larger surface-to-volume ratio of NR was demonstrated to increase the efficient surface area for detection, where the sensing elements were immobilized and therefore proportionally improved the sensitivity of the NR-FET biosensors [ 106 ].
Optical Biosensors.
Optical biosensors can detect luminescent, fluorescent, colorimetric, or other optical signals produced by the interaction of microorganisms with the analytes and correlate the observed optical signal with the concentration of target compounds. Optical biosensors have the biorecognition element integrated into an optical transducer system. They utilize enzymes, antibodies, aptamers, whole cells, and tissues as biorecognition elements. There are several types of optical biosensors based on optical waveguides, surface plasmon resonance (SPR), photonic crystals, and optical fibers [ 20 , 106 , 107 ]. Optical biosensors have been used for clinical diagnostics, drug discovery, food process control, and environmental monitoring.
Temperature Sensors.
These transducers determine the quantity of heat generated by biological material [ 31 ]. An interesting application of the temperature sensor is based on bimaterial cantilever beams, which were employed as highly sensitive temperature sensors for biological applications [ 108 , 109 ]. The bicantilever beam temperature sensor was fabricated from composite materials and operated in deflection mode. To achieve the high sensitivity required for the detection of heat generated by a single mammalian cell, the cantilever beam temperature sensor was microprocessed with a length at the microscale and a thickness in the nanoscale dimension [ 110 ]. As a thermogenic sample, the brown fat cells (BFCs) that are related to metabolic heat production were employed. The cantilever beam deflection was measured under a conventional microscope, when six cells of BFCs were situated about 5 µ m from the tip of cantilever beam, after the BFCs were thermal stimulated with flowing norepinephrine solution (NE). When the cantilever beam senses the heat, it bends due to the difference in the coefficient of the thermal expansion of the composite structure.
A second cantilever beam was included in a vacuum chamber and operated in the resonant frequency regime. The working principle of the vibrating cantilever beam temperature sensor is based on shifts in resonant frequency in response to temperature variations generated by mammalian cells [ 110 ]. The heat sensing of a single BFC cell was demonstrated using this resonant cantilever beam. The temperature measurements were performed by stimulating the activity of BFC by introducing NE solution (1 μ M) in the microfluidic chamber that contains BFC culture. The cells were introduced in the microfluidic channel and some cells spontaneously attached to the sample stage side of the cantilever beam. Then, resonant frequency measurements of the cantilever beam side situated in vacuum were performed. The heat production was observed a few minutes after adding NE, and the heat generation continued for approximately 23 min. A temperature change of 0.27 °C corresponds to 1 nJ of heat was measured.
Biosensors as analytical tools have several applications in different fields such as diagnostics, disease monitoring, and toxicity studies. The significant role of biosensors is illustrated in Table 1 , which presents several biosensors with transduction methods and applications. The applications of these sensors are interesting, due to the advances and discoveries in the technological areas. There are still numerous challenges of biosensors despite many advances in technology. In some biosensors, a large amount of data are generated quickly at the output, and the analysis of these data requires further processing by an experienced user which can lead to errors. Processing by a person can take time to analyze data, which can greatly reduce the efficiency of the biosensor.
Principal types of biological sensors with transduction methods and applications
| Sensor type | Transduction method | Applications | References |
|---|---|---|---|
| Electrochemical sensors | Voltammetry and amperometry | ELISA for detection of bone markers, DNA | [ , ] |
| Impedance spectroscopy (ECIS) | Monitor cell migration, wound repair, cell differentiation, cell attachment, cancer metastasis, cell viability, cell proliferation, cell apoptosis | [ , , , , , , , , , ] | |
| Piezoelectric sensors | QCM | Water toxicity, human skin odor, human urine analysis, cell adhesion viscosity properties of mammalian cell monolayer, detection of orchid virus, detection of avian influenza virus | [ , – , , ] |
| Acoustic sensors | SAW device | Immunosensing of Biomarkers, Heavy metal toxicity of water, marine toxin detection | [ , – ] |
| Optical sensor | Surface plasmon resonance (SPR) | Bacteria detection, Detection Biomarkers in oncology | [ , , ] |
| Temperature sensor | Biomaterial cantilever beam deflection and resonant frequency | Heat generated by BFC | [ – ] |
| Electric sensor | FET | AB fibrils, C-reactive protein (CRP) and carcinoembryonic antigen detection, prostate-specific antigen (PSA) | [ – ] |
Recently, machine learning (ML) has allowed for enhancement in analytical capabilities of these various biosensing modalities [ 114 ]. ML, a subset of artificial intelligence (AI), is a framework allowing algorithms to learn automatically from data. For medical applications, it has been shown that such methods can not only significantly outperform human-engineered expert knowledge, but they are also able to discover new knowledge.
Due to their nature and mode of operation, biosensors are designed with unique characteristics and features upon which their usability and reliability depend.
Sensitivity.
Sensitivity is the most important characteristic of a sensor. It is the detection limit, which is the minimal amount (or concentration) of analyte that can be detected. This characteristic shows the capacity of the sensor to capture any fluctuations occurring in the targeted analyte if it remains in the vicinity of the sensor. Highly sensitive sensors are affected by fluctuations at low scales such as nanogram and femtogram scales [ 107 , 112 , 115 ] has been reported that the sensitivity for glucose determination ranges from 0.048 to 3.36 mA L mol −1 cm 2 .
Selectivity.
Selectivity means that the sensor detects a certain analyte and does not react with added mixtures and contaminants. This characteristic of a biosensor is based on the ability to bind or communicate with the specific target analyte (molecule) in the presence of others in the same medium or test site. In implantable medical applications of biosensors, selectivity is one of the most important features of the device. This is because most of the analyte candidates in the bloodstream possess similar properties, and therefore, it is important that the bioreceptor part of the sensor communicate only with the analyte of interest [ 116 , 117 ].
The stability of a sensor refers to the signal drifting under constant conditions, which could cause errors. This feature of the biosensor ensures that it can withstand interference or noise from external factors during its operation. Noise in this case can be in the form of humidity that tends to affect the accuracy of the sensor signal in operation [ 118 ]. In addition, the temperature of the human body also impacts the effectiveness of the bioreceptor component of the sensor, thereby causing inconsistencies in the overall output of the sensor [ 119 ]. Other factors that affect the stability are the degradation of the bioreceptor over time and the affinity of the bioreceptor to the analyte.
Reproducibility.
Due to the delicate conditions under which biosensing is required, it is necessary that a biosensor produces consistent output results, under the same or similar conditions, using the same analyte. This ability to show repeatable results, whenever the sample is measured, is an important quality of the transducer [ 118 ]. Consistent calibration of the biosensors after use in accordance with the manufacturer's instructions will ensure and enhance reproducibility and consistency of results.
Response Time.
A biosensor's response time is the amount of time it takes to read and produce a signal after its bioreceptor meets the specific analyte [ 36 , 120 ]. For example, glucose oxidase–based sensors have a response time between 5 and 30 s [ 115 ].
Range or Linearity.
The linearity of a biosensor is its ability to exhibit variation in its output proportional to different analyte concentrations. This is used to determine the resolution of the sensor, that is the measurement of the minimum change in the concentration of the analyte that can generate a corresponding response from the sensor. This feature is useful when sensing a wide range of concentrations for a specified analyte [ 118 , 119 ].
For mass production, it is important that the biosensing parameters are quantitatively validated [ 121 , 122 ]. The analytic hierarchy process was used, to perform a quantitative analysis of the signal produced by a carbon nanotube tube sensor, that consisted of 9.8% noise, and 10.1% error from external factors, which means that only about 80% of the normalized signal was corresponding to the real signal [ 122 ].
This characteristic of a biosensor describes the minimum quantity and concentration of analyte that the sensor can detect in the sample. This is mainly used to determine the quality of the sensor, and it is for this reason that many describe it as the most critical feature in the design and selection of biosensors. It is often determined indirectly through a linear calibration function formulated from a linear regression performed on a set of measurements of instruments against the concentration of the analyte [ 123 ].
Biosensor Design Considerations.
In designing a biosensor, the principal thing is to identify and understand how the specific analyte of interest interacts with the bioreceptor (the component of the sensor that directly encounters the analyte). The following factors such as biological receptor selection, immobilization method, and transducer type must also be taken into consideration when designing a biosensor.
Biological Receptor Selection.
The biological receptor, being an important component of the sensor, is required to be highly sensitive and selective. These features enable it to (a) selectively interact with only the targeted biomolecule in the presence of others and (b) be able to capture the exact characteristics or behavior of the analyte. Understanding and acknowledging the advantages and disadvantages as well as the nuances in the biosensor applications play a major role in the selection and/or design of an appropriate receptor [ 124 , 125 ].
Immobilization Method Selection.
Immobilization is the process of depositing a biological molecule onto a surface, in this case, a transducer. Various methods/techniques are used in the process. In general, the choice of method used is primarily determined by the physicochemical properties of the surface under consideration [ 126 ]. Some of the methods include adsorption, entrapment, covalent attachment, microencapsulation, and cross-linking [ 31 , 127 ]. The most popular method used is adsorption because it is simple and inexpensive. The disadvantage is that the deposition done by this method is usually affected by experimental conditions like ionic strength, temperature, and pH due to the weak van der Waals forces forming the bonding [ 128 , 129 ].
Transducer Element Selection.
The sensitivity of a biological sensor greatly depends on its transduction method. In many applications, high sensitivity and low limit of detection are requested for monitoring a particular analyte of interest. However, in applications where selectivity is important to distinguish between the target analyte and other biological components, sensitivity could be affected. Therefore, finding a good balance between these features in designing or selecting a transducer element is indispensable [ 127 , 128 ].
A few decades ago, when in situ testing and monitoring of biological materials was not possible, samples were taken to labs for analysis. Aside from the fact that this practice provides scientists and engineers with enough time and a convenient environment for the necessary analysis, this tends to be quite expensive and time-consuming, and requires consistent recruiting and training of highly skilled experts to achieve the testing. With the development of biosensors, their applications are in different fields such as medicines, environment, food safety, defense, and drug discovery. Figure 8 illustrates different fields of application of biosensors.
![research papers on biosensors Different applications of biosensors. Reproduced with permission from Singh et al. [130].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f008.png?Expires=1726586656&Signature=mjM~kM~0hjUdK6BLMOHA-4dIWV60XYc7YQtp7JDlP2UCsoYCdz4U2LPXbhbPNeZN1-A2APEnGa0oOgTxQbR1vJ0BG6T3KsGANt23lCCvwzNwaOQXUhBkNnuDDqLE2drFuA2V-Owzc4YiIAvZBsG1WpBTzxKZyoT2qFBiTq2KXQDl~4iDUHC2zJ7mpUmPpxx0jVIYoXJncuY0VNam6jH4UpHS6nOVT1oVstxhyMeYRE6t8XOocbUt4eqoybDBcP3uLd6kdIVliQx1YqA-rd6WzbKjpEgK2Q3iac~LvacXox8cmkB2jxCAr78cClPrGlEMkZ~wOyshRNJatZ8sYyDNZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Different applications of biosensors. Reproduced with permission from Singh et al. [ 130 ].
Medical Applications.
For medical applications, biosensors are designed to interact with the biomolecules of the organism they are residing in. The application of biosensors in the medical field has experienced rapid growth over the years. This is due to the ease with which it has made diagnosis, testing, and monitoring of medical conditions in patients. For instance, Electrochemical biosensing systems coupled with Graphene Quantum Dots (GQDs) biosensors have been proven to enhance the early detection of cancerous cells, as well as monitor the biomarker concentrations of the affected area. This helps medical practitioners to differentiate between normal and cancerous cells [ 131 ]. Optical biosensors are also reported by Kaur et al. [ 132 ] as an efficient tool for diagnosing different types of cancers in real time owing to their low sensitivity and low detection limit. They are also used to monitor and control blood sugar levels, as well as monitor cardiovascular conditions in real time during and after surgical procedures. Other medical applications include diabetes control and pathogen detection [ 133 ]. It was reported by Bohunicky et al. [ 134 ] that biosensors can help in early detection of cancer. This can be achieved by constantly monitoring certain protein levels as well as secretions from tumors. This will go a long way to help mitigate the pain and subsequent death of a large number of people carrying the disease.
Cardiovascular disease is the leading cause of death globally. Hence, researchers and medical practitioners have developed various techniques, such as immunoaffinity column assays, fluorometric assays, and enzyme-linked immunosorbent assays for sensing, monitoring, and managing cardiovascular problems [ 135 – 137 ]. However, with the use of biosensors, these expensive and time-consuming techniques can be avoided in case of early detection, monitoring, and treatment of cardiac problems. The emergence of biosensors provides a significant number of advantages over conventional diagnosis assays mentioned because they are established on electrical measurements and employ biochemical molecular recognition elements with the desired selectivity with respect to a particular biomarker under consideration [ 138 , 139 ].
Environmental Applications.
With the adverse environmental pollution comes health problems for those in the immediate vicinity. As discussed prior, sensitivity and selectivity are key features of biosensors that could continuously scan the environment to sense the presence and the quantity of chemical agents, organic pollutants, potentially toxic elements, and pathogens that might pose a health hazard. Typical biosensors for environmental applications make use of antibodies, aptamers, nucleic acids, and enzymes as biological receptors. These biosensors include immunosensors, aptamer sensors, and enzymatic biosensors. Biosensors can also detect pollutants by measuring color, light, fluorescence, or electric current as reported by Refs. [ 140 – 145 ].
Application in the Food Industry.
In the food industry, biosensors are employed as quality assurance tools. They are useful from the crop-growing stage to the processing of the food. Biosensors are also used for automation in food processing facilities for sorting food and reducing the time and cost of this food process. Biosensors can detect low concentrations of different chemicals in food. Moreover, biosensors are used to detect, monitor, and quantify the contamination of food from other sources such as container surfaces [ 146 – 149 ].
Advancements in Biosensors Due to Nanomaterials Discovery.
Using nanomaterials, the sensitivity and performance of biosensors can be significantly improved [ 150 ]. Nanomaterials with at least one of their dimensions varying in a range from 1 to 100 nm display unique properties as compared to their bulk because their nanometer size enhances their physical properties (electrical, electrochemical, optical, and magnetic). Generally, the nano biosensors use nanomaterials in combination with microscale-size transducers.
The nanoparticles used for biosensors are nanoparticles with the capability of high detection sensitivity such as AuNPs, AgNPs, quantum dots (QDs), magnetic nanoparticles, nanowires, nanotubes or nanoribbons with very high surface area, nanomaterials with high electron conductivity such as carbon nanotubes (CNTs), Au nanowires, thin films at nanoscale such as graphene, polymeric materials as dendrimers, and photonic crystals (PC) [ 150 ]. An extensive presentation and classification of nanosensors showing their characteristics, detection mechanism, and application of biological nanosensors is given in Ref. [ 151 ]. Examples of nano biosensors’ applications include the environment as well as the industrial sites, detection of ultra-low concentrations of potentially dangerous substances, detection of biochemicals in cellular organelles and medical diagnosis, and monitoring of physical and chemical phenomena in humanly unfriendly environments [ 151 ].
The research for nanosensors started in the last decade, and the progress shown in this area has been remarkable. Currently, there is extensive research on the nanoscale phenomena, discovery of nanomaterials, and continuing progress in nanotechnology fabrication tools that will generate further achievements in the field of nanosensors. This can be achieved through the enhanced performance of existing nanosensors and the discovery of novel types of nanosensors based on novel nanomaterials and detection techniques. Table 2 presents the main types of nanomaterials used for nanosensors, transduction methods, and applications of biological nanosensors.
Nanomaterials-based biosensor transduction type and applications
| Nanomaterials | Transducer type | Biosensing application | References |
|---|---|---|---|
| Nanoparticle (NP) AuNPs, AgNPs, magnetic nanoparticles | Localized surface plasmon resonance (LSPR), ELISA | Medical imaging, drug delivery, protein and DNA detection, virus detection | [ , – ] |
| Nanorods | Surface plasmon resonance (SPR), electrochemical detection, FET | Protein and DNA detection, phosphate detection | [ – ] |
| Nanowire (NW) | Conductive transduction, FET, SPR | Disease diagnostics, Drug delivery, protein and DNA detection, immunoglobulin detection | [ – ] |
| Quantum dot (QD) | Resonance energy transfer (FRET) | Detection of organic compounds, pharmaceutical analytes, cancer biomarkers, virus | [ – ] |
| Carbon nanotubes (CNT) | Bioenzymatic electrochemical Biosensors, near-infrared (NIR) optical sensing, FET, conductive transduction | Glucose monitoring, fructose, galactose detection, DNA, and cancer biomarkers detection | [ , , ] |
| Dendrimer | Fluorescent sensing | Disease diagnostics, drug delivery | [ – ] |
Wearable Biosensors.
Wearable biosensors and smartwatch technologies have taken health, exercise, and sleep monitoring to a few notches higher in recent years. Their emergence has made the measurement of a combination of physiological data, such as heart rate, heart rate variability, respiration rate, and other vital signals easy to archive due to their user-friendliness. With wearable biosensors, the physiological conditions of the patients are continuously monitored in real-time [ 175 ], as illustrated in Fig. 9 . These wearable devices worn by patients enable medical personnel to take necessary actions before the effects of the change of the monitored parameters get dangerous. Modern optical and electrochemical-based wearable sensors are made of flexible electronics, wireless data transmission, and self-powering capability [ 177 , 178 ]. Gao et al. [ 179 ] have designed a fully integrated wearable sensor for monitoring perspiration to detect glucose, Na + , and K + in patients. Yetisen et al. [ 180 ] developed a minimally invasive dermal tattoo realized on the skin-based colorimetric biosensor, that is able to detect changes in glucose and pH. Another interesting idea is to detect glucose levels in the tears. Elsherif et al. [ 181 ] have developed a wearable contact lens-based sensor for monitoring real-time glucose levels. Several tattoo-based biosensors have been researched with relatively minimal invasion procedures. In this technique, different types of dyes that changed colors in response to pH levels and other stimuli were used [ 182 , 183 ].
![research papers on biosensors Illustration of a remote health monitoring system based on wearable sensors. Health-related information is gathered via body-worn wireless sensors and transmitted to the caregiver via an information gateway such as a mobile phone. Caregivers can use this information to implement interventions as needed. Reproduced with permission from Patel et al. [176].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f009.png?Expires=1726586656&Signature=CzFfe66CXGZNJCTQSWdvQPCSgpA37vBTPhuDV7CuEySarhhIVtqCmcWmkB6-M6UMoYEOOusYicpWw1osvWS4youm5pWqneAppnYJgcLi-zgqQYhaSuMRSQRKrraTk8HmUKUyQf-H2ABVCJnGzXLTcAVZrhcuS7eWZVjdiZDanaHQcqQ~2i517Dm-dGV2-s~IYWGqVYyU1ihHMdZfHNBrL5oOsf8NkHmXO2JFcRg4mtPLRJoNqkr1NhHh7iKbBit-cNU4ImdMxx5HvpGc5Sfm00u3H8686ucETHvrAhSUgLnvt4nlCn9k8-IJfOGCPSW~JvS6HmmfDGme5xBrO4OsHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Illustration of a remote health monitoring system based on wearable sensors. Health-related information is gathered via body-worn wireless sensors and transmitted to the caregiver via an information gateway such as a mobile phone. Caregivers can use this information to implement interventions as needed. Reproduced with permission from Patel et al. [ 176 ].
A low-cost and high-accuracy wearable piezoelectric-based sensor for blood pressure monitoring was also developed [ 184 ]. The piezoelectric sensor was mounted on the subject's wrist above the radial artery and was demonstrated as suitable for continuous, long-term blood pressure-monitoring applications.
Yapici et al. [ 185 ] took advantage of the impeccable conductive properties of graphene-clad textiles to develop a fully wearable, cloth-based smart medical garment for monitoring in real-time and for long periods of time the electrocardiogram (ECG) activity of the human body. Figure 10 illustrates the placement of the ECG sensors on the left and right hand and the recording of the electrical signal of the heart [ 185 ].
![research papers on biosensors Real-time demonstration of ECG measurement using the prototype graphene-clad textile embedded wearable wristband with integrated electronics. Reproduced with permission from Yapici and Alkhidir [185].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f010.png?Expires=1726586656&Signature=gOVhvk6AuWa9c9PcG~ylnnw6Me41ift~h~bTsRiIiOrw6L8q05UvIWmZnhVP24DW8DlJGSfQbFJ1tjX6g3HobvPdcjAw4wMzT0ME3v9y~5xgvVZ9u~IkFlG0AXvKolgocnhsxjsUuKIYOutR7YLUTHEw2UVKsEC8UrUz~qGxWztL9DwRoS2GggHKJkMOU4bnrZ2RFmPR4jP6pGY1TVYQFLiLAsczTMu5fac6mC5kvVwBtlGRemPSZjaBqEsoqw4cPlF3zKdu2U12CNGXTJP4Gckj2-miXUWu2V7mkGCYV0jG1uCPO4-CN3PlqWib6yYvDjlID9S2dg~ln8cryKyatg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Real-time demonstration of ECG measurement using the prototype graphene-clad textile embedded wearable wristband with integrated electronics. Reproduced with permission from Yapici and Alkhidir [ 185 ].
Piezoelectric ZnO was used for the fabrication of a wearable and stretchable piezoelectric power generator, that could harvest energy from the movements of the body (Fig. 11 ) [ 186 ]. The micro-fabricated power generator had a thickness in the micrometer scale to be attached to the skin or garments and stretched by the natural movements of arms, legs, or neck. This power generator demonstrated a maximum power output of 200 µ W. It is expected that energy harvested by this device could power wearable sensors [ 157 ]. Obviously, wearable sensors with their current capabilities and esthetic packaging features have come to stay, and they will continue to be the basis on which further biosensing devices and their applications will be developed.
![research papers on biosensors Image of the fabricated ZnO power generator. It is fabricated by a thin layer of ZnO with Au power collection electrodes fabricated on both sides of the ZnO film. The substrate of the stretchable power generator is PDMS [157]. Reproduced with permission from Voiculescu et al. [187].](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f011.png?Expires=1726586656&Signature=a2etwpbbP6cKu~~v9HbXlWF90GQjcOKXMehLqHpXcOCAy6i0puiEvK2FF5hzOJYjFtSwA35LL5dMPIIJsu1JPZC2SSlXYcc~LRdlpXfZiz3B-eHeBUWieT4DOkLbW6TAoa5ysrL2nh-gnWBriQWcAHEcv6hlfJTDQiTEnI2MjUv-2LePM8VPPI~G3gCTFK2OCAp7t2mVwPVBayxlVcY9zgfoj9pzDFDqdv-BSYkXhvl-55Ax9yb3XB-V8syGit7lhowHhRSHsUYhr9lUvmfQ0BLL2vTfc9xtQo9ro2N23JBF7MHwTu-J4AEbSu4We77RyFpkfq5olvCIBjBO42LoQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Image of the fabricated ZnO power generator. It is fabricated by a thin layer of ZnO with Au power collection electrodes fabricated on both sides of the ZnO film. The substrate of the stretchable power generator is PDMS [ 157 ]. Reproduced with permission from Voiculescu et al. [ 187 ].
Data processing and interpretation is an integral part of biosensing. Conventionally, mathematical models are the tools deployed in data processing in most biosensors. However, for multianalyte-based sensors, this technique has become limited due to their inability to process data beyond two and three dimensions [ 188 ].
This has led to the introduction of more sophisticated data processing techniques such as preprocessing and machine learning algorithms in modern biosensing technologies. Neural and non-neural network models are subdivisions of machine learning algorithms, typically used in the design of biomedical signals [ 188 , 189 ].
Data Preprocessing.
Like many data mining projects, careful cleaning of the raw data or signal received by a biosensor is critical in enhancing accuracy and efficiency. Typical data manipulation techniques used in the preprocessing stage are normalization, standardization, image analysis, transformation, as well as data metric synthesis [ 190 ]. The number and type of preprocessing techniques required largely depend on the nature of the raw data received.
Non-Neural Algorithms.
Several choices of non-neural ML algorithms are used in the processing of biomedical signals detected by biosensors. The most popular ones are Principal Component Analysis (PCA) and Linear discriminant analysis (LDA), and Random Forests (RFs), hierarchical cluster analysis (HCA), support vector machine (SVM), and decision trees (DTs). The schematic illustrations of these algorithms are shown in Fig. 12 .
![research papers on biosensors Schematic illustrations of machine learning algorithms. (a) PCA. Reproduced with permission [184]. Copyright 2016, Springer Nature. (b) SVM. Reproduced with permission [191]. Copyright 2018, Springer Nature. (c) HCA. Reproduced with permission [186]. Copyright 2015, John Wiley and Sons. (d) RFs of three trees. Reproduced with permission under the terms of the Creative Commons Attribution License [192]. Copyright 2014, The Authors. Published by Taylor & Francis.](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f012.png?Expires=1726586656&Signature=a54s~0Nwnm6jmqvMy2rXfnUBsrN487xlry4Xx2N0Z-SmBgJKztq1cj674bcczzLMtKZruwjtJeBd9ON0LhKkZTplCiLBZUtfCCTlYLtCySVQicwzKa3h1arxedJhbDWsKpqBiJdkGBdERghm0hbIQdct2uaq2Pmezr-xQeDDPzvEEEaA0E1OcDeIck7L7FZ6~Y2o1aZQ04LOg2gM4qhH0SWsJ3XjUMyWHOe45jAeoxuDGl9nxk0B2lRb9q7EZB4tG99HBKilpR9flEyP7FsjlJAS7-i98FrWTBY-6WFSRDfqZyprIYAWupyMrmgFQfMXrG0c0ck~Bpf5rKhuhx7dkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic illustrations of machine learning algorithms. ( a ) PCA. Reproduced with permission [ 184 ]. Copyright 2016, Springer Nature. ( b ) SVM. Reproduced with permission [ 191 ]. Copyright 2018, Springer Nature. ( c ) HCA. Reproduced with permission [ 186 ]. Copyright 2015, John Wiley and Sons. ( d ) RFs of three trees. Reproduced with permission under the terms of the Creative Commons Attribution License [ 192 ]. Copyright 2014, The Authors. Published by Taylor & Francis.
Neural Algorithms.
The Neural Algorithm is identical to the neuron network of living organisms, such as the human brain. This algorithm consists of a structured network of artificial neurons that are activated one after the other in a defined pattern [ 188 ]. The amount of data required to train a neural network is huge, and this is an inherent disadvantage as compared to the non-neural type of algorithm. In most cases, it is challenging to incorporate neural networks into biosensors for the purposes of data collection for physiological monitoring biosensors. Wearable and noninvasive biosensors have helped overcome the issue. Neural networks are subdivided into categories: (1) the Feedforward Neural Network (FNN), (2) Recurrent Network (RNN), and (3) Convolution Network (CNN). Figure 13 illustrates the latest architecture of the Neural Network [ 193 ].
![research papers on biosensors Schematic illustrations of the latest Neural Network Architecture [193]](https://asmedc.silverchair-cdn.com/asmedc/content_public/journal/openengineering/2/10.1115_1.4063500/2/m_aoje_2_020201_f013.png?Expires=1726586656&Signature=nd-u84DALoaTpmqUM2Ed6yUrPoC~I6~lroSYStjntTB8xVlGqdiE8nWP2nxo5K0aqx~EjIPu2jTkt9y3E2rTgXM4WF0Bizia5zsudEfBS8VWOnGKhSI8QFoiU7TDDq39o3O4KI9TuqoN8u9eyLlBQAWJkz2-Xyy05rV2jWwCVRFD25xTg1tMcJBw52704U52p51vg2RfMaGk1oSv0cLNRHq2sm9K5G2TryHmfiJVLMpQHelMWZjxrOGMyCiQ0I0ngoRHzvKYk9S7MISVoCr1YFO7iwImqKuiveC6dGQ2e5HcFqUFjKxZ15DSFEaRWrtPrb62Mict6gPSeb33jrgdhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic illustrations of the latest Neural Network Architecture [ 193 ]
In conclusion, the application of machine learning in biosensing technology, and its applications will certainly enhance the need to make them autonomous while improving their accuracy.
This review paper presented biosensors, their applications, and recent advancements in the use of Machine Learning techniques. Several types of biosensors with their transduction methods were discussed with a focus on electrochemical, thermometric, QCM and acoustic biosensors, and wearable biosensors.
Nanomaterials used for biosensing were also introduced. Nanomaterials became important components in bioanalytical devices since they were demonstrated to enhance the performance of biosensors in terms of sensitivity and detection limits down to single-molecule detection. The specific properties of nanomaterials could improve the performance of the classic transduction methods. The combination of different nanomaterials, each with different characteristics, could increase the performances of biosensors even more. Due to the vast number of different nanomaterials, all with their specific properties, only a few examples of nano biosensors were mentioned in this review paper to highlight the principal advantages of such materials. We also discussed biosensors based on nanomaterials, AI, ML, their challenges, and the potential to revolutionize the biosensors industry. Technological advancement in the field led to the development of wearable devices for continuous and real-time monitoring of physiological signals. Their successful applications depend less on technological issues and more on the challenges of practical ethical, legal, and social issues, regarding data collection and usage.
The biosensor industry is interested in integrating the miniaturized biosensors in personalized point-of-care (POC) devices, because of their reliable, sensitive, and fast detection of biomolecules with portable features. The recent advances in the camera and processor of smartphones are being integrated with biosensing applications, and this could increase the precision of healthcare diagnoses. Recently developed wireless technologies are better than their wired counterparts, but they require more innovation in the design of low-power ICs. IC fully integrated within the smartphone eliminates the need for external hardware and the processor unit, so there is a need for more fully integrated smartphone-based sensors for personalized health care. In conclusion, the combination of biosensors, nanomaterial-based technology, and smartphone technology can improve POC-based devices for better health care.
The authors wish to thank S.M. Mahbobur, Caroline Miko, Sarah Gudelis, Ward McHenry, Varun Modak, Arin Ofir, and Anna Taylor of the Spring 2022 ECE Senior Design team of Virginia Tech.
All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
No data, models, or code were generated or used for this paper.
Get Email Alerts
Related articles, related proceedings papers, related chapters, affiliations.
- Accepted Manuscripts
- About the Journal
- Editorial Board
- Information for Authors
- Call for Papers
- Rights and Permission
- Online ISSN 2770-3495
ASME Journals
- About ASME Journals
- Submit a Paper
- Title History
ASME Conference Proceedings
- About ASME Conference Publications and Proceedings
- Conference Proceedings Author Guidelines
ASME eBooks
- About ASME eBooks
- ASME Press Advisory & Oversight Committee
- Book Proposal Guidelines
- Frequently Asked Questions
- Publication Permissions & Reprints
- ASME Membership
Opportunities
- Faculty Positions
- ASME Instagram

- Accessibility
- Privacy Statement
- Terms of Use
- Get Adobe Acrobat Reader
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Sensors (Basel)

Recent Trends in Biosensors for Environmental Quality Monitoring
Associated data.
Not applicable.
The monitoring of environmental pollution requires fast, reliable, cost-effective and small devices. This need explains the recent trends in the development of biosensing devices for pollutant detection. The present review aims to summarize the newest trends regarding the use of biosensors to detect environmental contaminants. Enzyme, whole cell, antibody, aptamer, and DNA-based biosensors and biomimetic sensors are discussed. We summarize their applicability to the detection of various pollutants and mention their constructive characteristics. Several detection principles are used in biosensor design: amperometry, conductometry, luminescence, etc. They differ in terms of rapidity, sensitivity, profitability, and design. Each one is characterized by specific selectivity and detection limits depending on the sensitive element. Mimetic biosensors are slowly gaining attention from researchers and users due to their advantages compared with classical ones. Further studies are necessary for the development of robust biosensing devices that can successfully be used for the detection of pollutants from complex matrices without prior sample preparation.
1. Introduction
The modern world faces a major problem today—environmental pollution, which is caused by the release and accumulation of various harmful substances due to current industries’ extreme development, rapid urbanization, and population growth. Pollutants are very diverse, ranging from chemical to physical, biological, and radiological compounds, and are widely spread in the air, soil, and waters, affecting all living systems, especially human health and life [ 1 ]. The safety and security of the environment is a major concern worldwide; therefore, prudent monitoring and management of it constitute two of the global and European priorities [ 2 ]. Researchers are interested in finding durable solutions to environmental monitoring, as the control of toxic substances is a fundamental condition for pollution remediation. Usually, the classical chromatographic [ 3 , 4 , 5 ] and spectroscopic [ 6 , 7 , 8 , 9 ] methods are used to detect contaminants, which are generally characterized by high sensibility and selectiveness. However, these methods are laborious, need several sample preparation steps, use toxic chemicals, and are time-consuming; and the equipment needs well-qualified operators.
The necessity of using some rapid, selective, sensitive, accurate, and real-time devices for detecting and screening pollutants led to the development of advanced biosensing devices. These must combine the analytical techniques with biotechnology in careful and reliable ways, at a low cost [ 10 , 11 , 12 ]. A special use of biosensors is in the evaluation of ecological risks. Biosensors are in such cases essential in complementing the specific chemical analyses [ 13 , 14 ]. For the construction of the biosensors should be considered the complexity of the environmental samples, as their use for technological applications is highly demanded [ 15 , 16 , 17 ].
Environmental pollutants can be monitored using specific biosensors. The detection principle must be based on a suitable physical/chemical transducer integrated with a compatible biological or biomimetic element that reversibly binds the analyte. The detector identifies and converts the resulting reactions into qualitative and quantitative sensing signals for the targeted pollutants from the sample [ 11 , 16 ].
The pollutants released from industrial, agricultural, and other intense human activities [ 11 ] are organic and inorganic. Biosensors’ usage is essential for monitoring actual conditions of soil, water, and air samples to detect pollutants such as pesticides, potentially toxic elements, pathogens, toxins, and endocrine-disrupting chemical compounds [ 2 ]. The major and long-lasting environmentally relevant toxicants can be separated into four categories: organochlorine pesticides (aldrin, chlordane, DDT (dichlorodiphenyltrichloroethane), dieldrin, endrin, heptachlor, mirex, and toxaphene); fungicides (i.e., hexachlorobenzene); industrial chemicals (PCBs—polychlorinated biphenyls and their by-products), and heavy metals . The possibility of their quantification by using specific biosensors constitutes a significant advantage in controlling them [ 11 ]. Even though biosensors have proved their abilities to measure air pollutants in various sample types, their efficiency is often poor [ 10 ].
The capacity of these small devices to offer reliable analytical results productively and profitably should be highlighted [ 18 ]. Another characteristic that needs to be underlined is the possibility offered by to perform ongoing in-field monitoring of various pollutants [ 19 ].
Biosensors are analytical devices that each incorporate a biological sensing element to detect a targeted analyte from complex samples [ 20 ]. Biosensors convert a biological signal into a detectable electrical, optical, or thermal signal. They provide high sensitivity even with miniscule analyte concentrations [ 1 , 21 , 22 ]. A schematic diagram of the typical components of a biosensor is presented in Figure 1 .

Operation of a biosensor.
A biodetection device consists of some distinct components: a bioreceptor, a transducer, a system for signal processing, and a display [ 16 , 21 ]. The entire unit produces a measurable detection signal relating the analyte’s concentration in the target [ 23 ]. The biochemical receptor is used to recognize biological or chemical elements from the analyzed sample, being intimately associated with the transducing element, which converts the biochemical outcome into quantized electrical, optical, or thermal signal [ 21 , 22 , 24 ]. The biorecognition element might be a biological material, such as enzymes or a multienzyme system, microbes, recombinant microorganisms, functional nucleic acids, antibodies, antigens, aptamers, or an animal or plant tissue [ 21 , 24 ]. New alternatives use biomimetic materials (biomimetic catalysts, molecularly imprinted polymers, combinatorial ligands, etc.) [ 25 ]. Even if the biosensor is a complete, independent unit, the term specifically refers to the component that provides precise, complex bioanalytical measurements in simple formats and in real-time [ 10 , 20 , 24 ]. Biosensors must allow reuse and not be affected by pH and temperature [ 26 ].
Biosensors are classified by the most important components involved in the detection process: the bioreceptor and the transducer. Regarding the bioreceptor type, biosensors can be grouped as follows: the biocatalytic group (enzymatic biosensors), the bioaffinity group (immunosensors, aptasensors, genosensors), and the microbial group (microbial biosensors) [ 2 , 26 ]. Based on the transducer’s physicochemical features and its working principle, biosensors are categorized as: electrochemical (potentiometric, amperometric, impedimetric, conductometric biosensors), optical (fiber-optic, surface plasmon resonance, Raman spectroscopy-based, and FTIR-based biosensors), and mass-based (magnetoelectric and piezoelectric biosensors) ( Figure 2 ) [ 16 ].

Classification of biosensors.
Biosensors present some advantages in analytical chemistry. They expedite the processes of the traditional laboratory and analytical monitoring procedures—that is, taking various analytes from diverse samples. They are small and simple devices with high sensitivity and bioselectivity for targeted analytes, precision, rapidity, and continuity in monitoring. Several factors for users must also be considered when designing them, such as easy manipulation and operation, safety functioning, suitability for in situ detection (no complex sample preparation), real-time detection, cost efficiency, and eco-friendliness [ 27 , 28 ].
Biosensors have seen rapid and varied development in the past few decades [ 10 ] due to their ability to identify a wide range of analytes, such as pollutants, bacteria, fungi, drugs, and food additives [ 16 ]. Such attributes demonstrate their great applicability in various fields—pharmaceutics, medicine, industry, environmental monitoring, agriculture, food, forensic chemistry, security and defense, robotics, etc. [ 24 , 27 ]. The main uses of a biosensor depend on the specific tasks of the application area. Their utility in the food industry was demonstrated in quality and safety control, by discerning natural and artificial components, monitoring fermentation processes, etc. Their applicability in industry is mainly in control processes. In drug discovery and clinical and medical sciences, their use is recommended for rapidly detecting chemicals or viruses that cause various diseases, including cancer [ 20 , 26 ].
Currently, there is increasing interest in developing highly accurate and efficient systems for identifying and screening environmental pollutants ( Figure 3 ) [ 29 ].

Biosensors used for the environmental quality monitoring.
Compared to other types of biosensors, e.g., biomedical ones, biosensors for environmental monitoring have a nonaged phase due to the complexity of the analysis, such as the complex ecological matrix, which interferes with pollutant recognition.
A biosensor’s characteristics are directly related to its biorecognition element and its transducer’s properties. Therefore, the materials used for the construction of the biosensor play an important role. Recently, laminated composites have become of great interest to various industries and applications [ 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. The development of new composite materials is grabbing researchers’ attention, as these materials are characterized by high surface-to-volume ratios, high catalytic activity, good electrical conductivity, and good magnetic properties [ 43 , 44 , 45 , 46 , 47 ]. Yang et al. [ 47 ] extensively presented the synthesis of carbon nanotubes (CNT) (arc discharge, laser ablation, chemical vapor deposition (CVD), etc.) and the possibilities for their functionalization.
Nanocomposites represent a promising technology that enhances the sensitivity and flexibility of analyses of environmental complex samples. Nanostructures such as tubes, wires, rods, and particles modify biosensors’ characteristics toward achieving this goal. However, as Nigam et al. [ 10 ] noticed, there is still a real need for innovations in biosensors for environmental purposes, to assure high output of analysis for continuous, automated, and real-time results. Still, accuracy must also be considered the primary priority.
2. Sensors Used for Environmental Monitoring Overview
2.1. enzyme-based biosensors.
Enzymes are macromolecules with a complex 3D structure consisting of proteins that act as biological catalysts. An enzyme-based biosensor uses a specific enzyme as a biological sensing element, combined with a transducer that converts the signal generated by the enzymatic reaction into a measurable response proportional to the analyte concentration [ 48 ]. The enzymatic reaction signal can be generated in different forms: thermal release, proton concentration changes, oxygen emission or uptake, light emission or absorption, etc. The transducer (optical, electrochemical, thermal, piezoelectric) transforms this signal into potential, current, temperature exchange, light absorption, etc.—all of these being measurable by different means [ 49 ].
Enzymatic biosensors have earned massive interest in the last few years due to their multiple advantages, such as the high specificity and selectivity of enzymatic reactions, their wide range of detectable analytes, flexibility in detection, and the high purity of the available enzymes [ 50 ].
Naresh et al. [ 51 ] present in their paper the operating principles of enzymatic biosensors. There are two possible categories of mechanism of action: metabolization of the target analyte by the enzyme; or the activation, inhibition, or alteration of the enzyme by the analyte.
The essential requirements of an enzymatic biosensor are the immobilization the enzymes to the transducer’s surface and maintenance of their activity after immobilization [ 48 ]. The immobilized enzymes are more stable than the mobile versions and can be repetitively and continuously used [ 52 ]. The main methods for enzyme immobilization are presented in Figure 4 , and in Table 1 are the characteristics of these.

Methods for immobilization of the enzymes.
Methods of enzyme immobilization for biosensors [ 52 , 53 ].
| Immobilization of Enzymes | Method’s Characteristics |
|---|---|
| Adsorption | Simple, inexpensive, less destructive to enzymatic activity, no additional reagent necessary |
| Microencapsulation | Preservation of structural and acting integrities of enzymes, due to their protection against environmental conditions |
| Entrapment | High stability conferred to the enzymes |
| Cross-linking | Improved efficiency and stability of enzymes by strong and stable bindings |
| Covalent bondings | More stability for enzymes and enzymes-support complexes, meanwhile stronger bindings than in adsorption case |
Enzyme-based biosensors are widely used in food, medical, agricultural, and environmental fields. As shown in Table 2 , the development of enzymatic biosensors for environment monitoring represents a subject of considerable interest.
Examples of enzyme-based biosensors used for environmental monitoring.
| Analyte | Enzyme(s) | Immobilization Method | Transducer | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|---|---|
| Hg , Cu , Cd | Urease | Entrapment in sol-gel matrix | Optical | River water | 10 nM, 50 μM, 500 μM | - | [ ] |
| Chromium | GOx | Cross-linking with GA and covering with aniline membrane | Amperometric | Soil | 0.49 µg L | 0.49–95.73 mgL 95.73–8.05 mgL | [ ] |
| Paraoxon | AChE | Dropping on the multiwall carbon nanotubes | Amperometric | Water | 0.5 nmol L | 6.9 nM | [ ] |
| Paraoxon-ethyl, diisopropyl fluorophosphates | AChE | Cross-linking with BSA in a saturated glutaraldehyde vapor | Conductometric | Soil | 1 × 10 , 5 × 10 | - | [ ] |
| Atrazine | Tyrosinase | Cross-linking with PVA-SbQ | Amperometric | Spiked drinking water s | 0.3 ppm | 0.5–20 ppm | [ ] |
| Atrazine | Tyrosinase | Entrapping in poly(L-DOPA) | Amperometric | Water | 10 ppb | 50 ppb–3.0 ppm | [ ] |
| Organophosphorus neurotoxin | AChE | Cross-linking with GA | Piezoelectric | Water | 50 mg/m | 0–50 mg/m | [ ] |
| Captan | Glutathione-S-transferase | Entrapment in gel sodium alginate | Optical | Water | 2 ppm | - | [ ] |
| Anatoxin-a | AChE | Entrapment in PVA-SbQ | Amperometric | Water | 1 µg L | 0–2.0 ppm | [ ] |
| Catechol | Tyrosinase | Chitosan-gold nanoparticles | Amperometric | Environmental monitoring | 27 × 10 mM | 0.046–50 μM | [ ] |
| Methyl salicylate | Alcohol oxidase and peroxidase | Molecular tetherings in carbon nanotube matrix | Amperometric | Environmental monitoring | 0.00098 mM | - | [ ] |
Abbreviations: LOD—limit of detection; Gox—glucose oxidase; GA—glutaraldehyde; AchE—acetylcholinesterase; BSA—bovine serum albumin; PVA-SbQ—polyvinyl alcohol bearing styrylpyridinium groups; L-DOPA-l-3,4-dihydroxyphenylalanine.
2.2. Whole Cell-Based Biosensors (Microbial)
Whole-cell-based biosensors use natural or genetically engineered microorganisms (bacteria, fungi, algae, protozoa, or viruses) that can interact with a broad array of analytes and produce a signal detectable and quantifiable by a specific transducer [ 65 ]. Several transducers have been integrated with microorganisms, being built on different principles: electrical (amperometric, conductometric, potentiometric), colorimetric, and optical (colorimetric, luminescent, fluorescent) [ 66 , 67 , 68 ]. Microbial biosensors operate under a range of working conditions and are more sensitive to environmental signals than conventional ones [ 15 ]. They present various advantages: low limits of detection, high selectivity, and high sensitivity. Based on these features, whole-cell bioreceptors are applicable in many fields [ 51 ].
Microbial sensors can be considered a developed form of enzyme-based biosensors, as their mechanisms of detection are mostly identical. Both of them require the application of an immobilization technique to fix the biological material onto transducers or support matrices. As in the enzymes case, microorganisms can be immobilized by physical (adsorption and entrapment) and chemical methods (covalent binding and cross-linking). Finally, the chosen immobilization method must ensure mechanical resistance, cell viability, safe handling, and long-term storage [ 69 ].
Besides the advantages presented over the conventional methods, namely, high sensitivity, simultaneous detection of several compounds, high potential for on-site examinations, and cost-effectiveness, microbial biosensors are also associated with some drawbacks. Their long response times, the cells’ sensitivity to environmental variables (temperature, pH, etc.), and the difficulty of maintaining cell viability for an extended period are some of their limitations [ 15 , 65 , 70 ].
Numerous recent articles reported on the use of microbial biosensors to detect environmental pollutants, such as pesticides, heavy metals (As, Cu, Hg, Pb, or Cd), phenols, and other toxic compounds, using terrestrial and aquatic biota [ 15 , 19 , 71 , 72 ]. Other microbial biosensors were proposed and developed in the last few years as well, with remarkable applicability to environmental monitoring. Table 3 summarizes the results of several such investigations reported in the literature.
Examples of microbial biosensors used for environmental monitoring.
| Analyte | Microorganism | Immobilization Method | Transducer | Target | LOD | Reference |
|---|---|---|---|---|---|---|
| As | Genetically engineered | Biofilm formation | Electrochemical | Environmental monitoring | 40 μM | [ ] |
| Cu , Cd , Ni Pb | S288C | Physical adsorption on BND-chitosan hydrogell polymer on GCE | Amperometric | Wastewater | - | [ ] |
| As , Cd , Pb , Zn | Microbial culture in microfluidic device | Fluorescent | Water | - | [ ] | |
| Pb | DH5α | Microbial culture in a microfluidic device | Fluorescent | Environmental monitoring | [ ] | |
| Cd , Cu , Zn | VR1 | Entrapment in sol-gel matrix | Fluorescent | Soil | 1.42 × 10 , 3.16 × 10 , 2.42 × 10 | [ ] |
| Cu | Entrapment in alginate beads | Colorimetric | Water | 1 µM | [ ] | |
| Paraoxon, parathion, methylparathion | Genetically engineered | Biofilm on GCE modified with OMCs | Amperometric | Environmmental monitoring | 9 nM, 10 nM, 15 nMz | [ ] |
| Atrazine (herbicide) | Entrapment in alginate | Amperometric | Environmmental monitoring | 0.07 µM | [ ] | |
| Diuron (herbicide) | Ti/TiO ultramicroe-lectrodes in algal suspension | Chronoamperometric | Water | 0.2 µM | [ ] | |
| Simazine (herbicide) | Dc1M | Adsorption on porous silicone disks | Luminescent | Drinking water | 40.8 µg L | [ ] |
LOD—limit of detection; BND—boron-doped nanocrystalline diamond; GCE—glassy carbon electrode; OMCs—ordered mesopore carbons.
2.3. Antibody-Based Biosensors
Antibodies or immunoglobulins are a large class of glycoproteins produced by specialized cells as part of the immune system to detect harmful substances (antigens), such as microorganisms and chemicals. The antibodies can recognize and bind antigens, leading to stable antibody–antigen complexes [ 82 , 83 , 84 ]. Depending on how they are harvested, antibodies can be monoclonal or polyclonal. Monoclonal antibodies are laboratory-produced by hybridoma selection, whereas polyclonal antibodies are complex mixtures of antibodies isolated after animal immunization [ 85 ].
Antibody-based biosensors, also called immunosensors, are compact devices that detect and quantify, using a transducer, the specific interaction between immunoglobulins and antigens. Depending on the transducing mechanism, immunosensors are classified as electrochemical (amperometric, potentiometric, and impedimetric), colorimetric, optical, and microgravimetric. They can also be classified as labelled or nonlabelled sensors [ 17 , 86 , 87 , 88 ]. The labelling consists of attaching a sensitively detectable marker to the targeted analyte or the bioreceptor. During the analysis, the tag’s activity is measured. These tags may can be various sorts of compounds, including enzymes, fluorescent dyes, electroactive compounds, and nanoparticles [ 89 ]. Nonlabelled immunosensors are designed so that the antigen–antibody complex can be directly determined by estimating the physical changes produced by its development [ 51 ].
Immunosensors possess the advantages of better selectivity and sensitivity than classical analytical methods. At the same time, the evolution of immunoreactions on the detector’s surface can be observed in real-time [ 83 , 90 ]. However, the limitations in using antibody-based biosensors must also be considered, such as pH and temperature sensitivity, considerable time consumption, and the need for developing specialized reagents for each compound [ 91 ].
Several applications of the antibody-based biosensors within environmental monitoring are summarized in Table 4 .
Examples of immunosensors used for environmental monitoring.
| Analyte | Transducer | Electrode/Sensing Material | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|---|
| Chlorpyrifos | Impedimetric | Chip modified with gold nanoparticles | - | 0.5 ng mL | 0.5–500 ng/ml | [ ] |
| TBBPA-DHEE and TBBPA-MHEE | Impedimetric | Silica nanoparticles | Aquatic environments | 0.08 ng mL | 0.21–111.31 ng/mL | [ ] |
| Atrazine | Electrochemical | SWCNT | Seawater, riverine water | 0.01 ng mL | - | [ ] |
| Microcystin-LR | Impedimetric | Gold electrodes with MoS2 andgold nanorods | Water | 5 ng L | 0.01–20 gL | [ ] |
| Okadaic acid Domoic acid | Optical (SPR) | Gold electrode with carboxymethylated surface | Seawater | 0.36 ng mL 1.66 ng mL | - | [ ] |
| Okadaic acid | Impedimetric | Graphene | Seawater | 0.05 ng mL | - | [ ] |
| | Optical (SPR) | Gold substrate | Water | 103 CFU mL | - | [ ] |
Abbreviations: TBBPA-DHEE—tetrabromobisphenol A bis(2-hydroxyethyl) ether; TBBPA-MHEE—tetrabromobisphenol A mono(hydroxyethyl) ether; SWCNT—single-walled carbon nanotubes; SPR—surface plasmon resonance; microcystin—LR-microcystin-leucinearginine.
2.4. DNA/Aptamer-Based Biosensors
2.4.1. aptamer-based biosensors.
Aptamers or “chemical antibodies” [ 99 ] are artificial, single-stranded oligonucleotide (DNA (deoxyribonucleic acid) or RNA (ribonucleic acid) sequences (15–80 base pairs in length) that can bind to specific target molecules [ 100 ]. The range of aptamer targets is extensive, from small molecules (peptides, proteins, carbohydrates, metal ions) to cells, viruses, and bacteria [ 101 , 102 , 103 ].
Aptamers can be selected in vitro through a process called SELEX (systematic evolution of ligands by exponential enrichment) [ 104 , 105 , 106 ]. The SELEX procedure starts with preparing an extensive library of oligonucleotides with different sequences, with which the target molecules are incubated for some time. After incubation, unbounded molecules are separated, and the target-bound oligonucleotides are eluted by heating or washing. The bound aptamer molecules are amplified by the polymerase chain reaction (PCR) to create the input for the following selection rounds. The entire process uses 5–15 cycles of selection and amplification [ 107 , 108 , 109 ].
In comparison with antibodies, aptamers have some specific advantages, such as higher stability in various environmental conditions (temperature, pH), lower cost, the ability to regenerate, and the possibility of being chemically synthesized or modified in accordance with target molecules [ 89 , 102 , 108 ].
In the last few years, several biosensors (colorimetric, fluorescent, electrochemical, and SERS—surface enhanced Raman spectroscopy) have been designed to detect environmental pollutants, using aptamers as the bioreceptors. Furthermore, the synthesis of new nanomaterials showed their significant potential for the development of innovative aptasensors. The latter are sustained by their strong biocompatibility with aptamers [ 102 , 106 ].
Table 5 summarizes recent studies on aptasensors developed for the detection of pollutants.
Examples of aptamer-based biosensors used for environmental monitoring.
| Analyte | Detection Method | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|
| Ag | SERS based on Au@Ag core–shell nanoparticles | Tap water, river water | 50 × 10 mg L | 0.1–100 nM | [ ] |
| As | Colorimetric with GNPs | Wastewater | 0.0006 mg L | 1–400 range/ppm | [ ] |
| As | Colorimetric with AuNPs | Soil | 1.97 ppm | - | [ ] |
| Cd | Fluorescence with use of SYBR green I as signal reporter | Tap water, river water | 3 × 10 mg L | 1.12–224.82 μg L | [ ] |
| Hg | SERS based on dual recycling | Water environment | 0.11 fM | 0.2–125 fM | [ ] |
| Hg | SERS based on SiO @Au core/shell nanoparticles | Lake water | 10 × 10 mg L | - | [ ] |
| Pb | Electrochemical (Impedance), G-rich aptamer/MWCNTs/GNPs | Water | 4.3 × 10 M | 5.0 × 10 –1.0 × 10 M | [ ] |
| Pb | Fluorescence based on gold nanoflowers | Tap water | 0.285 nM | 0.01–850 nM | [ ] |
| Pb | Colorimetric with use of silver staining | Soil | 5.0 × 10 mg L | - | [ ] |
| Acetampirid | Chemiluminescence with use of AuNPs | Wastewater Soil | 62 × 10 mg L 1.0 × 10 mg L | - | [ ] |
| Malathion | Colorimetric based on AuNPs and cationic polymer | Lake water | 6 × 10 mg L | 0.5–1000 pM | [ ] |
| Omethoate | Fluorescence based on S-GQD | - | 1 ppb | 0–200 ppm | [ ] |
| Organophosphorus pesticides | Fluorescence with poly(T) CuNPs | Lake water | 0.22 nM | 0–200 nM | [ ] |
| Tetracycline | Photoelectrochemical based on CdTe-BiOBr heterojunction | Soil | 9.25 pM | 10–1500 pM | [ ] |
Abbreviations: GNPs—gold nanoparticules; G—guanine; SERS—surface-enhanced Raman scattering; CuNPs—copper nanoparticles; S-GQD—sulphur-doped graphene quantum dot, SYBR—N′,N′-dimethyl-N-[4-[(E)-(3-methyl-1,3-benzothiazol-2-ylidene)methyl]-1-phenylquinolin-1-ium-2-yl]-N-propylpropane-1,3-diamine; G-rich—guanine-rich; MWCNTs— carboxylic acid group functionalized multiwalled carbon nanotubes (MWNTs-COOH).
2.4.2. DNA-Based Biosensors
DNA-based biosensors use nucleic acids (single-stranded DNA, ss-DNA) as recognition elements. Their working principle is based on two mechanisms: (i) the hybridization process between the target DNA and its complementary strand immobilized on a sensing area through the spontaneous hydrogen bonding between adenine–thymine and cytosine–guanine pairs [ 49 , 124 ]; (ii) the alteration of the ss-DNA structure by the target analyte’s molecules [ 125 ]. These mechanisms induce various physicochemical changes that lead to the generation of a specific signal that can be converted into a measurable response by an appropriate transducer, usually optical or electrochemical [ 126 ].
A significant stage in the design of DNA-based biosensors is the immobilization procedure of the nucleic acid fragments on the electrode surface. Regardless of the method used (adsorption, covalent bonding, or avidin–biotin interaction), the immobilization must preserve the activity of these fragments—that is, ensure their stability and accessibility to the target molecules [ 127 ].
Due to their multiple advantages, such as specificity, sensitivity, biocompatibility, and cost-effectivity, DNA-based biosensors are used in several fields, including disease prognosis, clinical diagnosis, food control, and environmental screening [ 126 , 128 ].
Several studies have illustrated the ability of DNA-based biosensors to detect traces of heavy metals in the environment [ 125 , 128 , 129 , 130 ]. In this case, the working principle is based on the affinity of some heavy metal ions toward forming stable duplex structures together with certain DNA bases. Mercury ion (Hg 2+ ) selectively binds thymine (T) bases and creates a thermal stable T-Hg 2+ –T duplex [ 131 ]. Similarly, silver ions (Ag + ) selectively interact with two cytosine (C) bases and form C–Ag + –C base pairs, which stabilize the DNA duplex [ 49 , 125 ]. Therefore, in the presence of some metal ions, thymine-rich or cytosine-rich single-stranded DNA can form stable structures by which metals can be detected with adequate transducers [ 125 ].
Some of the recent DNA-based biosensors’ applications are presented in Table 6 .
Examples of DNA-based biosensors used for environmental monitoring.
| Analyte | Transducer | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|
| Hg | Electrochemical | Tap water, river water | 0.05 nM | 0.1–200 nM | [ ] |
| Pb | Fluorescent | Aqueous systems | 5 nM | 0–50 nM | [ ] |
| Pb | Fluorescent | Lake water | 0.6 nM | 2–10 nM | [ ] |
| Organophosphorus pesticides | Fluorescent | Lake water | 0.018 µg L | 2–10 μg/L | [ ] |
| Cyanazine | Impedimetric | Water | 0.8 nM | 4.0 nM–70 μM | [ ] |
| Pirazon | Impedimetric | Water | 1 × 10 M | 5 × 10 –5 × 10 M | [ ] |
| Optical (SPRi) | Water | 104 CFU mL | - | [ ] | |
| Impedimetric | - | 7.41 × 10 mol L | 10 –10 mol L | [ ] | |
| Amperometric | Soil | 100 cells/g soil | - | [ ] | |
| Impedimetric | - | 0.997 × 10 M | 1 pM–1 μM | [ ] | |
| cf. | Colorimetric | Plankton, bentonite | 9 pg/μL | - | [ ] |
Abbreviations: SPR—surface plasmon resonance imaging; CFU—colony-forming units.
3. Biomimetic Sensors
Although the terminology may seem new, the basis of biomimetics was laid years ago. Its principle is finding solutions that mimic a natural system’s mechanisms, especially regarding the structure of an organism or its specific interactions with the environment. The created products can be performant and adequately adapted to real environments [ 142 ].
Biomimetic sensors were first constructed while considering the basic principles of the related enzymatic biosensors. The intention was to maintain high sensibility, selectivity, sensitivity, and easy operation, while simultaneously decreasing some of the disadvantages. The limitations that need to be overcome mainly relate to each enzyme’s specific features, such as inactivation issues, or high costs because of the purification and standardization processes. In such contexts, the research was conducted toward finding sustainable solutions for creating imitative systems. Some of the developed models are based on metal complexes, molecularly imprinted polymers, nanozymes, synzymes, and nanochannels [ 143 ].
In the last few years, the domain of biomimetic sensors has registered significant progress. Initially, biomimetic sensors were constructed using uni- or bi-dimensional structures ( Figure 5 ). Then tridimensional assemblies were widely used, and the results indicated improved performances, sometimes exceeding the natural models’ performances [ 143 ]. Finding the proper ligand for the targeted analyte is the first step in designing precise tools. The peptide selection used in the recognition systems is important for the sensor’s affinity [ 144 ]. Computer modelling [ 145 ] and simulation are two stages that improve the performances of these devices.

Structures used for the construction of biomimetic sensors.
The domain of biomimetic sensors used for environmental pollutants detection is currently developing. Research has opened multiple promising directions for the construction of such sensors: modified nanoparticles [ 146 , 147 , 148 ], metal chalcogenides nanocrystals built on various microorganisms [ 149 ], valorization of classical imprinted electrodes [ 150 ], and nanozymes for phenol removal [ 151 ].
Some examples of sensors created based on mimetic principles with applications in environmental monitoring are summarized in Table 7 .
Examples of biomimetic sensors used for environmental monitoring.
| Analyte | Mimetic Structure | Transducer | Target | Sensibility (LOD) | Linearity | Reference |
|---|---|---|---|---|---|---|
| Heavy metals | ||||||
| Cu , Cr , Fe , Pb , Fe , Cd , Cr , Co , Zn , Ag , Al | Enzyme immobilization Metal phosphates-acetylcholinesterase nanoflowers | Colorimetric | Water | Cu —0.81 μM, Cr —0.75 μM Al —1.06 μM | 2.5–500 μM. | [ ] |
| Pb | Gold nanoparticles with glutathione linker | UV–vis spectroscopic | Water | 47.6 nM (9.9 ppb) | 2–14 mM | [ ] |
| Hg | Cysteine-decorated ferromagnetic particle (Cys-Fe O ) | Colorimetric | River water | 5.9 pM. | 0.02–90 nM | [ ] |
| Chemicals | ||||||
| Methyl green | Magnetic molecularly imprinted polymer | Square-wave adsorptive anodic stripping voltammetry | River waterIndustrial wastewater | 1.0 × 10 mol L | 9.9 × 10 –1.8 × 10 mol L | [ ] |
| Acetylcholinesterase inhibitors | Microchannel 1-phenyl-1,2,3-butanetrione 2-oxime (PBO)-based microsensor | Potentiometric | Surface waters used for municipal drinking water supplies | LD50, LC50 | 2–1360 mg kg | [ ] |
| Acetone gas | Zeolitic imidazolate framework-90 polyhedron crystals | quartz crystal microbalance | Air | Lower than 20 ppb | - | [ ] |
| Nitrite ions | Oxo-bridged dinuclear manganese-phenanthroline complex immobilized into an ion-exchange Polymeric film deposited on glassy carbon electrode | Cyclic voltammetry | Environmental samples | 6.50 × 10 mol L | 2.49 × 10 –9.90 × 10 mol L | [ ] |
| Catechol | Metal-organic frameworks | Water | 33 nmol L | - | [ ] | |
| Urea | Embedding urease and bovine hemoglobin in metal-organic frameworks through biomimetic mineralization | Colorimetric | Sewage | 0.02 mM | 0.08–20.00 mM | [ ] |
| Pesticides | ||||||
| Diurone | Carbon paste electrode modified with the nickel(II) 1,4,8,11,15,18,22,25-octabutoxy-29 ,31 -phthalocyanine complex | Cyclic voltammetry and amperometry | River water, soil | 6.14 × 10 mol L , | 9.9 ×10 and 1.5 × 10 mol L | [ ] |
| Organophosphorus pesticides | Employing a functionalized polyacrylamide, polyhydroxamicalkanoate | Amperometric | Water supply | 0.26 μmol L | - | [ ] |
| Carbamate | Gold nanoclusters-anchored MnO (AuNCs-MnO ) nanocomposite | Fluorimetric/Colorimetric | Soil, water | 0.125 µg L . | - | [ ] |
| Paraoxon | Cu (PO ) ·3H O, AChE and ChO -based lab-on paper platform | Cyclic voltammetry and Colorimetric | Tap and river water | 6 fg mL | - | [ ] |
| Toxins | ||||||
| Bacterial toxins | Microcystins inserted into a polymeric matrix | Potentiometric | Water | below the guideline value establishedby WHO | 7.24 × 10 –1.28 × 10 M | [ ] |
Abbreviations: LOD—limit of detection; LD50—lethal dose (50%); LC50—lethal concentration (50%); WHO—World Health Organization; Cys—cysteine.
4. Future Perspectives
Another approach of biosensors regards the possibility of simultaneous detection of multiple pollutants. Several investigations have been successfully conducted to that end. Raymundo-Pereira et al. [ 164 ] evidenced the possibility of using carbon screen-printed electrodes for parallel identification of estradiol, paracetamol, and hydroquinone in tap water. Their findings could have an important application in wastewater analysis. Good prospects for use in water quality analysis were also provided by a luminescent sensor derived from a stable europium(III) metal–organic framework. It was tested for antibiotic identification [ 165 ]. The interest in using biosensors for water contaminant detection was also fostered by Martins et al. [ 166 ]. They identified sulfamethoxazole and trimethoprim from water samples.
The first steps toward making a biosensor with two detection mechanisms were made by Belaidi et al. [ 167 ]. Their electrochemical and optical detection biosensor, based on different algae responses, showed promising perspectives for simultaneous pesticide identification in water samples. These findings also provoked the design of a mimetic biosensor capable of detecting multiple pollutants.
The biosensors constructed for environmental quality monitoring will continue to be improved by using novel nanocomposites and nanomaterials, and new functionalization methods, but the necessity for in situ and real-time monitoring of pollutants will lead to the development of new sensing systems and even their coupling with aircraft systems [ 168 ].
With the current need for cheap, sensitive, fast, and reliable devices for environmental monitoring, the main challenge remains the gap between the results of academic research and the implementation of these biosensors as marketable products.

5. Conclusions
This review aimed to show that the need for fast, reliable, and stable devices for the detection of environmental pollutants can be satisfied by biosensors. However, these should answer the demands of sensitivity and selectivity when used in complex and unpredictable environmental samples with changeable compositions.
Independent of the sensing element or transducer, when developing biosensors for environmental pollutants detection, it is important to consider the possibility of continuous use, which would require fast renewal of the biological activity during the detection cycles; portability; cost; and last but not least, the possibility of automatization and integration into professional devices. In most investigations, the performance of the biosensor is assessed based on standardized laboratory samples.
The biological sensing elements—enzymes, aptamers, DNA, antibodies, and microorganisms—might face challenges in terms of stability, possible interference, and optimal working conditions, but these still have the advantage of being open to improvements in terms of specificity and selectivity.
As a result of scientific research in recent years, biomimetic sensors are characterized by better kinetic performances than enzyme-based biosensors. Still, specificity and selectivity remain their main shortcomings.
Author Contributions
All authors conceived the review. S.P.-C. and C.Ș.U. wrote the Introduction and Section 2 . S.G. wrote Section 3 . F.-D.M. wrote the Future Perspectives and Conclusions sections and improved and corrected the paper. The authors made equal contributions. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 24 February 2023
Device integration of electrochemical biosensors
- Jie Wu ORCID: orcid.org/0000-0003-1379-122X 1 na1 ,
- Hong Liu ORCID: orcid.org/0000-0002-9841-1603 2 na1 ,
- Weiwei Chen 1 , 3 ,
- Biao Ma 2 &
- Huangxian Ju ORCID: orcid.org/0000-0002-6741-5302 1
Nature Reviews Bioengineering volume 1 , pages 346–360 ( 2023 ) Cite this article
37k Accesses
158 Citations
30 Altmetric
Metrics details
- Diagnostic markers
Electrochemical biosensors incorporate a recognition element and an electronic transducer for the highly sensitive detection of analytes in body fluids. Importantly, they can provide rapid readouts and they can be integrated into portable, wearable and implantable devices for point-of-care diagnostics; for example, the personal glucose meter enables at-home assessment of blood glucose levels, greatly improving the management of diabetes. In this Review, we discuss the principles of electrochemical biosensing and the design of electrochemical biosensor devices for health monitoring and disease diagnostics, with a particular focus on device integration into wearable, portable and implantable systems. Finally, we outline the key engineering challenges that need to be addressed to improve sensing accuracy, enable multiplexing and one-step processes, and integrate electrochemical biosensing devices in digital health-care pathways.
Electrochemical biosensors are self-contained, analytical devices, in which a biological recognition element is in direct contact with an electrochemcial transduction element to allow the sensitive and specific detection of analytes.
Depending on the design and sensor type, health-related and disease-related biomarkers, such as carbohydrates, proteins, nucleic acids and cells, can be rapidly analysed in different body fluids, including blood, saliva and tears.
Electrochemical biosensors, including amperometric, voltammetric, potentiometric, organic electrochemical transistor, photoelectrochemical and electrochemiluminescent sensors, can be integrated into wearable, portable and implantable devices to enable point-of-care diagnostics and health monitoring.
Commercialization and broad point-of-care applicability of integrated electrochemical biosensors will require improvements in stability, sensitivity, reproducibility, multiplexing, and digitalization and, importantly, low-cost materials and easy fabrication methods.
Similar content being viewed by others
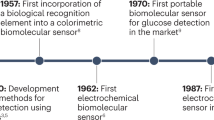
Biomolecular sensors for advanced physiological monitoring

Creation of a point-of-care therapeutics sensor using protein engineering, electrochemical sensing and electronic integration

A versatile bioelectronic interface programmed for hormone sensing
Introduction.
Biosensors have been widely applied in clinical, industrial, environmental and agricultural analyses since Leland Clark Jr introduced the amperometric glucose enzyme electrode in 1962 (ref. 1 ). According to the definition by the International Union of Pure and Applied Chemistry 2 , a biosensor is a self-contained, integrated, analytical device, in which a biological recognition element (biochemical receptors, including enzymes, antibodies, antigens, peptides, DNA, aptamers or living cells) is retained in direct spatial contact with a transduction element (such as electrochemical, optical and mechanical transducers). Biosensors were initially developed for point-of-care (POC) testing of biomolecular targets in the hope of extending clinical analysis from specialized laboratories to public settings, including hospitals, non-hospital nursing settings or home settings 2 . Although various biosensors have been developed for the sensitive and selective detection of a range of disease-related molecules, clinical translation of biosensors remains limited owing to difficulties in integrating and miniaturizing biosensors into portable devices.
Among the different biosensing platforms, electrochemical biosensors, which integrate the biorecognition element in an electrochemical transducer (for example, an electrode or field-effect transistor) are particularly suitable for device integration 3 , 4 , 5 (Fig. 1a ) because they can be easily miniaturized, batch fabricated and integrated with an electronic acquisition module on a single chip. In addition, electrochemical signals, such as electrical current and potential, can be collected by simple, portable and low-cost peripheral instruments with low power consumption. Moreover, the signal produced through affinity recognition of the target analyte by the biorecognition element can be amplified by physical, chemical or biological strategies, which greatly improves detection sensitivity. As such, electrochemical biosensors hold great promise for the development of POC diagnostic devices. The World Health Organization stipulates that POC biosensors should be affordable, sensitive, specific, user-friendly, rapid, robust, equipment free and deliverable to end users to enable on-site testing and diagnosis in the daily routines of individual patients and consumers. Thus, POC diagnostics are expected to play a key role in revolutionizing the diagnosis and treatment of major global diseases. For example, the electrochemical glucose meter, the most successful commercial POC biosensing device, has been widely used across the globe to help patients with diabetes.
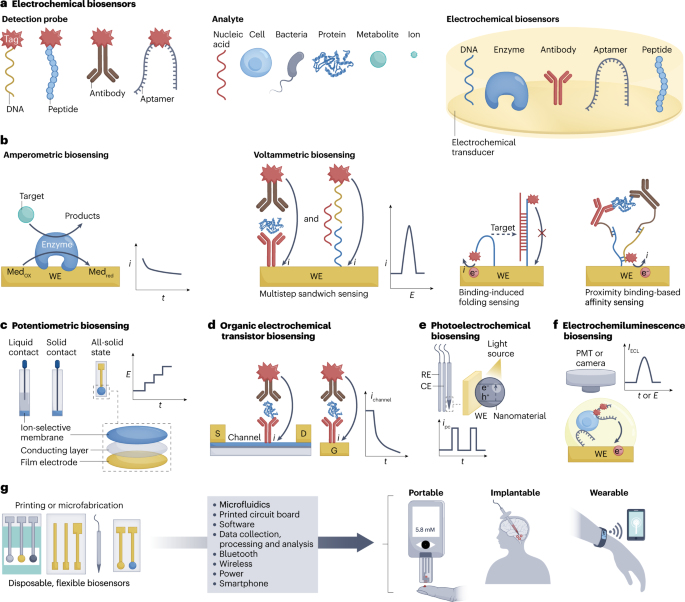
a , Schematic representation of electrochemical biosensors based on different biochemical receptors and detection probes. b , Amperometric biosensing of metabolite targets based on an enzyme electrode, including the current–time ( i – t ) curve and the i signal for quantification. Voltammetric biosensing of proteins or nucleic acids using an antibody-modified or nucleic acid-modified electrode through multistep sandwich sensing, one-step binding-induced folding sensing or one-step proximity binding-based affinity sensing, including the current–potential ( i– E ) curve and i signal for target quantification. c , Ion-selective electrodes with three different structures, including recording of the potential ( E ) for target quantification. d , Two types of organic electrochemical transistor sensors prepared by immobilizing the recognition element on the channel surface or on the gate electrode (G) for sandwich immunoassays of proteins, including recording of the channel current ( i channel ) for target quantification. e , Photoelectrochemistry biosensing based on a three-electrode system and a light source, including recording of the photoelectrode photocurrent ( i pc ) upon target recognition for quantification. f , Electrochemiluminescence biosensing of cells based on an aptamer-modified electrode through a sandwich-sensing format, including light intensity ( I ECL ) at excited potential by a photomultiplier tube (PMT) or imaging using a camera for target quantification. g , Integration of electrochemical biosensors in portable, wearable and implantable devices. CE, counter electrode; D, drain electrode; Med ox , oxidized form of mediator; Med red , reduced form of mediator; RE, reference electrode; S, source electrode; WE, working electrode.
However, the market for electrochemical biosensing devices is currently limited to the detection of some small molecules or ions (Supplementary Table 1 ), which can be detected directly by electrochemical signals through oxidation, reduction or affinity interactions at the electrode surface. By contrast, the detection of large biomarkers, such as proteins, nucleic acids, bacteria or cells, mainly relies on affinity recognition and, thus, requires multiple steps to produce a detectable signal. Several transduction principles may promote the integration of fully automated electrochemical biosensing devices for affinity biomarkers, including the relation of affinity recognition events with the generation and consumption of glucose 6 , 7 , 8 , 9 , 10 , one-step affinity-sensing mechanisms, such as binding-induced folding sensing and proximity binding-based affinity sensing 11 , 12 , and integration with automatic fluidic systems such as pump-assisted fluidics, paper-based microfluidics and polydimethylsiloxane (PDMS)-based microfluidics 13 , 14 , 15 . In addition, owing to the low concentration of disease markers in body fluids, in particular, in the early stages of disease (femtomolar or attomolar level), signal amplification strategies are required to increase detection sensitivity, which can be achieved by implementing nanotechnology-based and biotechnology-based strategies, such as amplification strategies based on nanotags, nanocatalysis, and nanocarriers and assembly-based and polymerase-based DNA amplification strategies 16 , 17 .
Efficient health-care management requires electrochemical biosensors to achieve minimally invasive or non-invasive continuous measurement of physiological molecules. Advances in microelectronic engineering, semiconductor precision-machining, flexible and stretchable bioelectronics and wireless communication technologies have spearheaded the integration of electrochemical biosensors in wearable and implantable devices 18 , 19 , 20 , 21 . To achieve long-term detection of molecules in different biofluids (for example, cerebrospinal fluid, interstitial fluid, sweat, saliva, tears and urine), electrochemical biosensors can also be integrated into flexible films, textiles, glasses, teeth and diapers. Furthermore, combining sensors with smartphones and other mobile devices allows continuous monitoring of dynamic physiological processes, and the integration of intelligent or digital processing modules into devices enables the connection of sensors to the Internet of Things and cloud computing for large-scale medical data mining.
In this Review, we discuss key innovations in electrochemical biosensing for preventive and personalized POC diagnostic devices. We discuss the design and integration of amperometric, voltammetric, potentiometric, organic electrochemical transistor (OECT), photoelectrochemical and electrochemiluminescent biosensors (Table 1 ) for disease diagnosis, health management, cell monitoring and neuroscience. In addition, we examine the fabrication, fluidic manipulation, signal amplification and readout, signal processing algorithms, and result visualization of integrated biosensors.
Electrochemical sensing of biomarkers
In electrochemical biosensors, the signal is typically triggered by electron or ion transfer on a conductive transducer through a biorecognition process. Signals can involve current ( i ), potential ( E ), impedance, conductivity, capacitance and light ( I ). Among these, impedimetric biosensors are theoretically favoured for POC diagnostics and device integration because they can directly detect biorecognition events by measuring the non-faradaic resistance and capacitance properties of the sensing electrode; however, their practical implementation suffers from non-specific binding of non-target compounds, which leads to low sensitivity and selectivity. In addition, although impedimetric biosensors have been greatly improved during the COVID-19 pandemic, for example, using molecular imprinting technology to fabricate virus-imprinted impedimetric biosensors for sensitive detection of whole virus particles 22 or by applying a dielectrophoresis force to improve the detection sensitivity of impedimetric immunosensors fabricated on Au micro-interdigitated electrodes 23 , their proof-of-concept performance is often only demonstrated using artificial physiological samples instead of clinically relevant samples.
Amperometric and voltammetric biosensors
Amperometric and voltammetric biosensors are operated with a three-electrode system, which contains a biosensor as a working electrode (WE) for target recognition, a counter electrode as the current source, and a reference electrode to apply a stable potential. Current signals are generated by electrochemical reactions on the WE under an applied potential for target quantification. The difference between the two techniques is their applied potential, which is constant for amperometric measurements and variable for voltammetric detection. According to the potential change modes, the latter can be performed with various techniques, including cyclic voltammetry, differential pulse voltammetry, square wave voltammetry and anodic stripping voltammetry.
Amperometric biosensors are the most popular sensors for the detection of metabolites (for example, glucose, lactate and uric acid). In amperometric biosensors, a target-specific enzyme (for example, glucose oxidase (GOx), lactate oxidase or uricase) is immobilized on the WE to catalyze the oxidation of the target at a constant potential 3 ; for example, glucose meters are typically constructed with amperometric biosensors that use GOx to catalyze the oxidation of glucose by a redox mediator (for example, ferricyanide, ferrocene derivative and transition-metal complexes) (Fig. 1b ); alternatively, amperometric glucose sensors can rely on the enzymatic oxidation of glucose with natural oxygen to generate and detect hydrogen peroxide using a mediator such as Prussian blue 24 . Amperometric biosensors are simple to fabricate, and have high sensitivity and selectivity in target detection, making them suitable for wearable applications. As the concentration of metabolites in non-blood fluids is lower than that in blood (for example, the concentration of sweat glucose (10–200 μM) and tear glucose (0–2 mM) are, respectively, 100-fold and 10-fold lower than that of blood glucose (1–20 mM)), nanomaterials, such as metallic nanoparticles, carbon nanotubes and graphene, can be added to the biosensing interface to facilitate electron transfer to increase the sensitivity and decrease the detection limit 25 . For example, Au–Pt bimetallic nanocatalysts in combination with nanoporous hydrogels enable GOx immobilization and glucose detection with a sensitivity of 180 μA cm –2 mmol –1 and a detection limit of 0.01 mg dl − 1 (0.56 μM), making such a biosensor suitable for integration with a smart contact lens for tear glucose measurement 26 . In addition, nanomaterials with enzymatic properties (that is, artificial nanoenzymes) can be implemented in amperometric biosensors to avoid denaturation of natural enzymes; for example, using a laser-induced graphene array, co-decorated with Cu 2 O and Au nanoparticles, a miniaturized, electrochemical, flexible, non-enzymatic biosensor was designed, offering stable sensing signals upon bending back-and-forth 25 times; its integration with a smartphone-based portable station for glucose monitoring has been verified with commercial blood testing devices 27 .
Metabolites can be detected by enzymatic recognition; by contrast, disease biomarkers, such as proteins and nucleic acids, are detected by affinity recognition, which cannot directly generate electron transfer on the biosensing surface. Thus, an additional electroactive label is needed for target sensing such as enzymes (for example, horseradish peroxidase and alkaline phosphatase), nanomaterials (for example, nanoparticles, nanotubes and quantum dots) and electroactive molecules (for example, ferrocene and methylene blue). These labels are typically detected by cyclic voltammetry, differential pulse voltammetry, square wave voltammetry and anodic stripping voltammetry, and therefore, most affinity sensors are voltammetric biosensors. Such sensors can be fabricated by immobilizing capture biomolecules (for example, antibodies, antigens, aptamers and DNA) on the WE to enable the detection of proteins or nucleic acids using a sandwich assay format (Fig. 1b ). Here, sequential incubations with the target, detection molecules and electrochemical nanotags are required 3 , 4 , limiting device integration of affinity biosensors. To simplify this operation, automatic fluidic systems can be applied; for example, biosensors fabricated on screen-printed carbon electrodes can be coupled with a flow-injection system to automatically detect multiple protein biomarkers 28 , 29 , 30 . However, the large size and high cost of this apparatus may limit commercialization.
Microfluidics allows the manipulation of fluids in micrometre-scale channels by integrated fluidic control units such as microvalves, pumps and reactors 31 . Therefore, multistep liquid processing workflows can be integrated into a single chip for fully automated sample-to-answer analysis 32 , 33 . Coupling electrochemical biosensors with microfluidics enables continuous and high-throughput detection of multiple trace analytes in complex samples such as human serum and blood samples. Several electrochemical biosensing devices have been commercialized (for example, Cue Reader from Cue Health, ePlex RP2 from GenMark Diagnostics, Binx io from Binx Health) for chip-based or cartridge-based detection of SARS-CoV-2 nucleic acids, respiratory viral and bacterial organisms, and Chlamydia trachomatis based on the integration of digital microfluidics (such as electrowetting) with amperometric or voltammetric affinity biosensors (Supplementary Table 1 ). These products enable at-home testing of disease biomarkers but are very expensive. Alternatively, cheaper and highly sensitive electrochemical biosensing systems can be designed by combining amperometric or voltammetric biosensors with paper-based microfluidics with self-pumping ability. These systems can integrate multiplex sensing electrodes for differential pulse voltammetry or square wave voltammetry detection of protein and nucleic acid biomarkers 13 , 15 . For example, an origami paper-based aptamer and antibody biosensing chip enables simultaneous detection of C-reactive protein (CRP) and pre-albumin down to the picogram per millilitre level 34 . By integrating amperometric or voltammetric biosensors with PDMS-based microfluidics, which can be fabricated by high-precision micromachining technologies, including soft lithography, casting, imprinting, injection moulding and laser ablation, the automation, miniaturization and array size of devices can be improved 35 , 36 , 37 . For example, 16 three-electrode biosensors integrated with a PDMS chip with separate chambers and reservoirs of reagents and samples allow the high-throughput detection of three protein markers of breast cancer 38 .
Merging biosensors with automatic fluidic systems simplifies detection; however, device integration remains difficult owing to the requirement of pumps and reservoirs. One-step affinity sensors provide a simpler alternative; for example, a binding-induced folding electrochemical biosensor can be fabricated by site-specific modification of a redox-tagged probe DNA on the WE 11 . The detection of the target then relies on the binding-induced change in rigidity of the probe DNA (Fig. 1b ), causing the redox tags to move close or away from the electrode surface, resulting in a respective increase or decrease of the current signal for target biosensing. Such binding-induced folding electrochemical biosensors can achieve sample-in-answer-out sensing of nucleic acids or sensing of some specific proteins using aptamer receptors; however, they suffer from low sensitivity. The signal can be amplified using DNA hybridization strategies 39 , 40 ; for example, an electrochemical DNA sensor based on target-induced CRISPR–Cas12a cleaving of interfacial single-stranded DNA with methylene blue as the signal tag can detect human papillomavirus 16 (HPV16) and parvovirus B19 (PB19) down to the picomolar level 41 . The sensitivity of this DNA sensor can be further improved using a hairpin DNA probe 42 .
Proximity binding-based affinity electrochemical biosensors are particularly suited for protein biomarker detection because they can transfer a protein immunoassay to DNA detection 12 . In such biosensors, a pair of antibody-DNA affinity probes dually recognizes a target protein, which leads to the formation of proximity ligation products that initiate DNA assembly, causing the ‘on’ or ‘off’ state of the electroactive molecule-tagged probe DNA on the electrode surface (Fig. 1b ). A wash-free and separation-free square wave voltammetry biosensor based on a proximity binding-induced ‘on’ state of methylene blue–DNA on the electrode surface allows direct detection of insulin 43 . By introducing uracils in the DNA sequence, this biosensor can be made reusable, enabling repeated protein quantitation within 3 min (ref. 44 ). The sensitivity of proximity binding-based affinity electrochemical biosensors can be further improved by DNA amplification strategies; for example, introducing an electrochemical ratiometric readout 45 , 46 , nuclease-mediated or DNAzyme-mediated cycle amplification 47 , 48 , surface programmatic chain reaction 49 , or DNA walker amplification 50 enables the one-step detection of glycoprotein markers (for example, carcinoembryonic antigen (CEA), prostate-specific antigen (PSA) and thrombin) down to picogram per millilitre or sub-picomolar levels (Supplementary Table 2 ).
Potentiometric biosensors
Potentiometric biosensors are typically operated with a two-electrode system consisting of a sensing electrode and a reference electrode, allowing direct detection of targets by measuring the potential signal related to the change of surface charge upon target recognition on the sensing electrode. Typically, ion-selective electrodes made of ion-selective membranes and a liquid contact structure are used as potentiometric sensing electrodes (Fig. 1c ). Glass membrane ion-selective electrodes (for example, pH electrode), solid membrane ion-selective electrodes (for example, crystalline membrane electrodes for F − , Ag + , Cl − and S 2− ), and liquid membrane ion-selective electrodes (for example, electrodes based on ionophores (selective host molecules) for H + , K + , Na + , NH 4 + , Ca 2+ ) are commercially available. Solid and liquid membrane electrodes can further be integrated into clinical analyzers for the detection of blood electrolytes (for example, Na + , K + , Ca 2+ , H + and Cl − ). Enzymes, nucleic acids and proteins can be detected by integrating the biological element on the ion-selective electrode to catalyze the reaction that forms the ions or by combining the target biorecognition event with an ionic reaction 51 , 52 , 53 , 54 .
Solid-contact ion-selective electrodes, which can be made with solvent polymeric membranes, do not contain internal solutions (Fig. 1c ) and benefit from ruggedness (thus, morphological diversity) and easy fabrication, modification and miniaturization. Solid-contact ion-selective electrodes allow protein and nucleic acid analysis through the detection of ions released from nanoparticle-tagged probes; for example, a miniaturized solid-contact Ag ion-selective electrode can detect DNA targets at the femtomolar level in microlitre-volume samples 55 . In addition, all-solid-state ion-selective electrodes can be made with conducting polymers or nanomaterials to establish a solid contact beneath the ion-selective and reference membranes (Fig. 1c ). Such ion-selective electrodes have been implemented in two commercial portable devices for POC detection of electrolytes and blood gases (i-STAT from Abbott and BGA-102 from Wondfo Biotech) (Supplementary Table 1 ). A paper-based potentiometric biosensor based on an all-solid-state butyrylcholine-sensitive ion-selective electrode and a 3D origami paper-based fluidic system can detect butyrylcholinesterase activity and organophosphate pesticides and, by further integrating a USB-controlled miniaturized electrochemical analyzer, allows the design of a handheld potentiometric device 56 . All-solid-state ion-selective electrodes can also be integrated into wearable devices for ionic detection in biofluids 18 , 19 , 20 , 21 ; for example, a wearable ‘smart wristband’ with Na + and K + ion-selective electrodes on a flexible sensing array enables in situ analysis of Na + and K + in sweat 57 .
Organic electrochemical transistor biosensors
OECT biosensors are organic thin-film transistors that consist of gate (G), drain (D) and source (S) electrodes, with an organic semiconductor film between the D and S electrodes. A change in the potential drop or capacitance of the gate–electrolyte or channel–electrolyte interface sensitively changes the channel current. Thus, OECT biosensors can be fabricated by immobilizing the recognition element on the G electrode or on the channel surface (Fig. 1d ); here, the specific reactions of the OECT biosensor with the target influence the interface potential, resulting in a channel current response for target quantification.
OECT biosensors benefit from high sensitivity, low cost, flexibility, easy fabrication and low working voltage (<1 V), allowing the detection of both electroactive (for example, dopamine, glucose and epinephrine) 58 , 59 , 60 and electro-inactive (for example, cortisol 61 , DNA 62 , proteins 63 , 64 , bacteria 65 , cells 66 and glycans 67 , 68 , 69 , 70 ) molecules or biomacromolecules through electrostatic interactions or affinity binding between targets and the sensing interface 71 .
OECT biosensors can be easily miniaturized, integrated into devices and designed as arrays because their detection performance does not degrade if their size is reduced at a fixed channel width per length ratio. For example, a ‘lab on a chip’ system based on an OECT biosensor integrated into a flexible microfluidic system allows label-free detection of DNA with a detection limit of 10 pM; here, the microfluidic device is deposited on a flexible substrate that contains a thiolated DNA probe immobilized on the Au gate electrode 62 . OECT microarrays can also be fabricated by solution processes for high-throughput sensing. The flexibility and robustness of OECT biosensors make them suitable for the non-invasive detection of biomolecules in wearable devices. For example, a fabric OECT biosensor, fabricated by weaving the sensor with cotton yarns, can be embedded in a diaper to monitor glucose in artificial urine, with the sensing signals collected on a mobile phone through Bluetooth 72 .
Photoelectrochemical biosensors
Photoelectrochemistry studies the effect of light on photoelectrodes or interfacial materials and the conversion of light energy into electrical power. Photoelectrochemical biosensing combines photoelectrochemistry with sensor-based bioanalysis; here, light serves as the excitation source and current as the readout. Photoelectrochemical biosensing systems typically consist of a three-electrode system and a light source (Fig. 1e ). Detection is based on the change of photocurrent upon target recognition at the biosensor surface, which induces a charge or energy transfer owing to the photoelectrochemical reaction between an electron donor and acceptor, and a photoactive material on the electrode surface upon light irradiation 73 .
Photoelectrochemical biosensors combine the advantages of optical and electrochemical assays, in particular, for the detection of disease-related molecules such as glutathione, lactate, DNA, microRNA (miRNA), protein tumour markers and cells 73 . Light stimuli can be applied contactless rather than through bias voltage, making photoelectrochemical biosensors biocompatible and suitable for in vivo sensing. In addition, separation of the excitation source (light) and detection signal (electricity) and their different energy forms result in low background noise and high sensitivity. Therefore, photoelectrochemical microbiosensors allow in vivo or single-cell analysis 74 , 75 ; for example, using a fluorescence resonance energy transfer (FRET) process, a photoelectrochemical microbiosensing system can selectively monitor SO 2 , a potential marker of cerebral ischaemia (reperfusion) and related brain injury, in the brain of living rats 76 . Here, FRET is implemented based on upconversion nanoparticles (UCNPs) as the energy donor and an organic dye as the energy acceptor. The biosensing interface is then constructed by co-immobilization of the UCNP and dye FRET pair, and CdTe quantum dots on a microelectrode. In the brain of a rat model of cerebral ischaemia-reperfusion and febrile seizure, the presence of SO 2 blocks the FRET process and recovers UCNP emission, which, in turn, modulates the photocurrent of the photoactive material, allowing the detection of SO 2 .
Electrochemiluminescence biosensing and bioimaging
Electrochemiluminescence is an electrochemically triggered energy-relaxation process, in which a luminophore undergoes electron transfer reactions to form excited states that emit light. Electrochemiluminescence biosensing enables the quantitative detection of target molecules through electrochemiluminescence emission signals that are associated with a target biorecognition-induced change in electrochemiluminescence active species. Similar to amperometric and voltammetric biosensors, electrochemiluminescence biosensors also operate with a three-electrode system, in which the WE is modified with the recognition element to serve as the biosensing electrode (Fig. 1f ). Owing to the combination of electrochemistry and spectroscopy, electrochemiluminescence biosensing does not require a light source and has negligible background noise, high sensitivity, good reproducibility, and high spatial and temporal control, making it a powerful analytical tool for the detection of a range of disease molecules, including DNA, miRNA, proteins and tumour cells 77 , 78 , 79 .
A commercialized microbead-based electrochemiluminescence biosensing system (that is, Elecsys 1010/2010/E170, Roche Diagnostics) is used as the gold-standard detection system in hospitals for many glycoprotein tumour markers 79 ; however, this instrument is large and bulky. Alternatively, a portable electrochemiluminescence device, integrating a screen-printed carbon electrode-based electrochemiluminescence biosensor, paper microfluidics and a mobile phone camera, can detect 2-(dibutylamino)-ethanol and NADH 80 . A portable electrochemiluminescence biosensing system has also been designed for the detection of miRNA-21 by combining a magnetic bead-based switch-on electrochemiluminescence molecular beacon sensing strategy with a portable potentiostat and a mobile phone camera readout 81 .
Electrochemiluminescence biosensing strategies can be combined with a charge-coupled device camera and a conventional microscope for electrochemiluminescence bioimaging. This system allows simultaneous detection of multiple biomarkers through spatial or potential resolution; for example, a bead-based electrochemiluminescence immunosensing array enables simultaneous detection of three antigens by individually imaging the microbeads located in a microwell array 82 . Similarly, electrochemiluminescent polymer dots (Pdots), luminol-doped Pdots and diethylamine-coupled Pdots can be exploited for potential-resolved and colour-resolved electrochemiluminescence bioimaging for the high-throughput detection of miRNAs 83 . Here, luminol-doped Pdots show blue electrochemiluminescence emission at +0.6 V, whereas diethylamine-coupled Pdots show red electrochemiluminescence emission at +1.0 V. On the sensing array, the electrochemiluminescence of two Pdots is initially inhibited by quencher-labelled capture DNAs. After recognition of target miRNAs, the quencher is released through DNA cleaving, and the electrochemiluminescence of Pdots is recovered for target detection. Compared to potential-resolved electrochemiluminescence biosensors, this potential-resolved and colour-resolved bioimaging system prevents interference of the threshold produced by the low potential emitter at high potentials.
Electrochemiluminescence bioimaging is well suited for cell analysis because it can provide both morphological and quantitative information 84 . Electrochemiluminescence cell bioimaging strategies have been developed for different targets, including small molecules released from cells and membrane proteins on the cell surface. Electrochemiluminescence imaging of membrane proteins is typically achieved by labelling cells with electrochemiluminescence probes through affinity reactions. However, this approach only allows observation of the cell periphery in contact with the electrode or requires membrane permeability treatment. Alternatively, a dual-intramolecular electron transfer strategy can be applied; for example, co-reactant-embedded Pdots with strong electrochemiluminescence emission enable in situ imaging of the membrane protein human epidermal growth factor receptor 2 (HER2) on single living cells 85 . To quantify detection, the biosensing interface, that is, a Pdot-modified-indium tin oxide (ITO) glass electrode sheet, can be combined with a single-cell-capture microfluidic chip, enabling high-throughput quantification of dopamine secreted by a single cell 86 .
Device integration
Electrochemical biosensors can be integrated into portable, wearable or implantable devices (Table 1 ), including microfluidics, printed circuit boards, software, signal processing units, communication units and power units (Fig. 1g ). Amperometric biosensors are the most developed and most commonly used sensors for metabolites. Owing to the specific enzyme reaction, they usually exhibit good selectivity. In addition, the enzymatic catalytic signal can be further enhanced by nanomaterials, leading to high sensitivity. Most importantly, these enzyme sensors can be prepared in batches with good reproducibility; however, enzyme activity can be affected by the environment. Thus, robust sensing electrodes are required for work in different environments. Potentiometric biosensors can be integrated for wearable sweat monitoring, in particular, for the detection of electrolytes. Using ion-selective membranes, potentiometric biosensors show good selectivity, reproducibility and stability; however, their sensitivity is low. Alternatively, a flexible, all-solid-state, wearable, ion-selective electrode could achieve continuous sweat monitoring. Voltammetric, OECT, photoelectrochemical and electrochemiluminescent biosensors allow the detection of proteins and nucleic acids, showing good selectivity and high sensitivity. However, such affinity biosensors typically require the specific assembly of bioreceptors on the electrode surface, making their fabrication more complicated than that of enzyme electrodes.
Portable electrochemical biosensing devices
Portable electrochemical sensors have initially been developed for the monitoring of blood glucose levels in patients with diabetes 87 . The personal glucose meter is a portable electrochemical biosensor that provides rapid quantification of blood glucose levels for personal glycaemic control. The glucose meter, which is typically an amperometric biosensor based on a redox enzyme, consists of a disposable test strip and a pocket-sized handheld electrochemical reader (Fig. 2a ). The disposable test strips can be fabricated by printing and cutting at a large scale using low-cost materials such as plastics and conductive pastes; for example, the thin-film electrodes on the test strips can be produced by screen-printing technology, which allows mass production at low cost 88 . The sensing layer containing the enzyme and the electron mediator is immobilized on the WE for the detection of glucose. Once the blood sample is introduced to the small chamber (electrochemical cell) formed by the spacer layer on the test strip, blood glucose is oxidized by the redox mediator, which is catalyzed by GOx (Fig. 2a ). The reduced mediator is then oxidized on the electrode, producing a measurable current signal 89 , which is converted to glucose concentration by a handheld detector. The personal glucose meter is a result of continuous engineering advances to increase its accuracy, reliability, user-friendliness and affordability 90 , 91 since the first concept of glucose enzyme electrodes was proposed in the 1960s 1 .
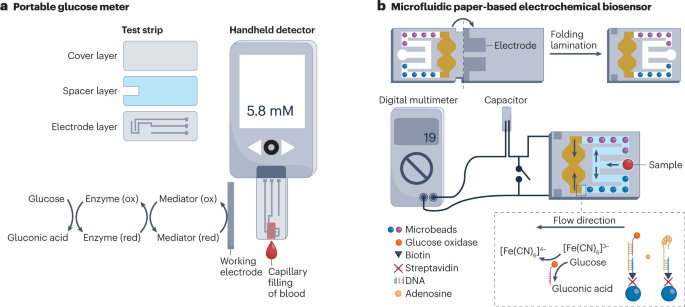
a , Portable blood glucose meter consisting of a handheld electrochemical detector and disposable test strips. The test strip contains a bottom electrode layer, an adhesive spacer layer and a hydrophilic cover layer. The blood sample is introduced to the reaction chamber by capillary force. b , A paper-based microfluidic electrochemical biosensor for the detection of adenosine through aptamer-based affinity sensing. In one channel, adenosine is recognized by aptamer-functionalized microbeads (blue), resulting in the release of glucose oxidase-labelled DNA to catalyze the oxidation of glucose, which leads to the conversion of [Fe(CN) 6 ] 3− to [Fe(CN) 6 ] 4− . In the other channel, the microbeads are not functionalized (purple), allowing quantification of adenosine concentration. The current signal from the discharging of the capacitor is collected by a portable digital multimeter. ox, oxidation; red, reduction. Part b reprinted with permission from ref. 104 , Wiley.
The personal glucose meter can also detect metal ions, drugs, organic metabolites, enzymes, proteins, DNA and influenza viruses by relating target recognition events with the generation or consumption of glucose 92 , 93 , 94 , 95 , 96 , 97 . For example, the personal glucose meter can quantify cocaine, adenosine and uranium in blood through the target-induced release of invertase, from a DNA–invertase conjugate, that catalyzes the conversion of sucrose to glucose 92 . Moreover, the device can quantitatively detect SARS-CoV-2 antigen in human saliva for COVID-19 screening 97 ; here, antigen-binding events are translated into glucose signals using an aptamer-based competitive mechanism that leads to invertase release to catalyze sucrose hydrolysis. This on-site test can be accomplished within 1 h with a picomolar limit of detection.
For complex samples that require pre-treatment, signal amplification and continuous analysis, electrochemical biosensors can be combined with microfluidic systems 98 , 99 , for example, for the detection of SARS-CoV-2 RNA 100 . In this device, RNA is detected by a reconfigurable enzyme–DNA nanostructure, which comprises DNA strands with inhibitor and inverter sequences that are bound to a Taq DNA polymerase through a cascading molecular circuitry enhancement; here, the biorecognition of target RNA by inverter sequences activates polymerase activity for downstream DNA amplification, labelling and electrochemical detection. The entire assay is automatically completed by a pressure-actuated microfluidic device with embedded sensing electrodes. Electrochemical biosensors can also be integrated with paper microfluidic devices by directly printing electrodes on paper. Paper-based microfluidics is cheap, biodegradable, easy to fabricate and allows pumpless fluidic transport by capillary actions 101 , 102 . In addition, paper can be folded (origami) to assemble 3D devices and control fluidic and electrical connectivity for programmed analytical processes 103 , 104 , 105 ; for example, paper with patterned fluidic channels and electrodes can be assembled into a 3D configuration by folding and lamination for the detection of adenosine 104 (Fig. 2b ). In this device, the adenosine sample is first split into two symmetrical channels. In one channel, adenosine binds an aptamer immobilized on microbeads, which causes the release of GOx-labelled DNA and leads to the conversion of [Fe(CN) 6 ] 3− to [Fe(CN) 6 ] 4− . In the other channel, the microbeads do not contain the aptamer, leading to different redox concentrations in the cells, allowing the quantitative analysis of adenosine in a portable digital multimeter.
Miniaturized electrochemical analyzers can also be connected to smartphones for powering, processing and storage of data and to display results 106 . In addition, the test results can be uploaded to mobile health services 107 . For example, an open-source portable electrochemical detector that can establish wireless communication with a smartphone can be combined with electrochemical biosensors 106 , 108 .
Integration into wearable devices
Wearable sensors can be integrated with smartwatches, bracelets and glasses for physiological monitoring, for example, of heart rate, electrocardiogram and electroencephalogram 109 . Such wearable biosensors also allow non-invasive and continuous monitoring of analytes in body fluids (Fig. 3a ), providing invaluable data for diagnostics and health management 109 , 110 , 111 . For example, the concentrations of glucose in non-blood body fluids, such as sweat and tears, can be converted to their corresponding blood levels through a correlation coefficient obtained from a correlation study between glucose concentration in blood and non-blood biofluids 26 , 112 , considering time lags for glucose secretion in different biofluids 113 , 114 , 115 . Compared to portable electrochemical biosensors, of which some have already been commercialized (Supplementary Table 1 ), wearable electrochemical biosensors are not yet at the same development stage.
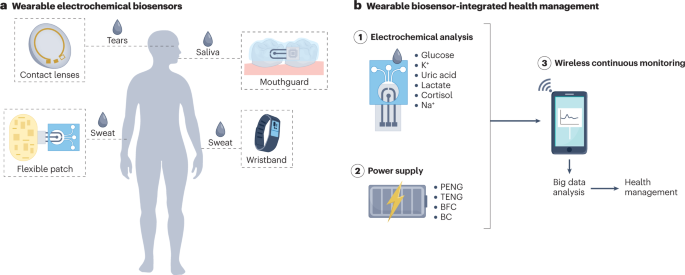
a , Wearable sensors can be applied to monitor health-related or disease-related analytes in different body fluids, including tears, saliva and sweat. b , Health management can be based on continuous monitoring using wearable devices, including electrochemical biosensors, power supply and wireless communication modules. BC, biocapacitor; BFC, biofuel cell; PENG, piezoelectric nanogenerator; TENG, triboelectric nanogenerator.
Thus far, wearable electrochemical biosensors have mainly been explored for glucose monitoring because glucose can be detected in sweat, saliva and tears 116 , 117 , 118 . Compared to the conventional finger-prick test, wearable glucose analysis allows non-invasive and continuous monitoring, even during sleep, enabling timely feedback for diabetes management. A wearable integrated sensing array allows multiplexed detection of sweat biomarkers, including metabolites and electrolytes (such as glucose, lactate, Na + and K + ); here, signal conditioning, processing and wireless data transmission for in situ sweat analysis are achieved by flexible printed circuit boards 57 . Such integrated wearable electrochemical biosensors allow non-invasive and dynamic monitoring of the health status at the molecular level, for example, for in situ monitoring of wound healing 119 , therapeutic drugs, drug abuse 120 , nutrition 121 and the diagnosis of cystic fibrosis 114 . Wearable electrochemical biosensors can also be incorporated into robots to sense hazardous materials and pathogens for agriculture, security and public health applications 122 .
Sampling plays an important role in wearable biosensing. The concentration of biochemical analytes in secreted body fluids is affected by various factors, including reabsorption, evaporation, secretion rate, interfering substances and metabolism of the secretion glands 109 , 113 , 123 . Microfluidic devices can be applied for sample collection; for example, sweat can be enriched and transported to a sensor module in a microfluidic device, reducing sweat reabsorption and evaporation, and allowing real-time continuous monitoring. Moreover, a microfluidic sweat sampling device can be designed to collect small volumes of sweat, enabling continuous sweat monitoring at rest by entrapping thermoregulatory-generated sweat in a microfluidic channel 124 . This design may facilitate wearable sweat sensing platforms that do not require large sweat volumes, for example, during exercise or at high ambient temperatures, making sweat sensing compatible with daily activities. Microfluidic devices with fluidic valves further allow in situ manipulation of collected biofluids, for example, to achieve chrono-sampling of sweat for time-dependent analysis of biomarker variation 125 . An epidermal microfluidic device with thermo-responsive hydrogel valves enables active control of sweat 126 , that is, on-demand delivery of sweat to the sensing electrode, thereby eliminating the influence of flow rate variability on the sensor response and allowing scheduled sweat analysis. Although promising for on-body biofluid detection, electrochemical bioassays in this device remain difficult because they require multistep operations for incubation, amplification and washing, limiting its use to monitor protein and nucleic acid biomarkers in sweat. Therefore, innovative fluidic control units are needed to automate multistep bioassays.
A power source is indispensable for continuous electrochemical analysis in wearable devices. Self-powered devices can generate energy from human motion 127 , 128 , 129 using a piezoelectric nanogenerator 130 , 131 or a triboelectric nanogenerator 132 , 133 , 134 that converts mechanical energy into electrical energy. For example, a self-powered wearable device based on a triboelectric nanogenerator printed on a flexible circuit board enables continuous monitoring of H + and Na + in sweat 135 ; here, the output of the power source (~416 mW m –2 ) can power the multiplexed biosensor and the design allows miniaturization. A triboelectric self-powered sweat sensor based on nanocellulose hydrogels with self-healing ability can monitor ions (Na + , K + , Ca 2+ ) in sweat 136 . Alternatively, biofuel cells can power wearable biosensors by harvesting energy from redox substances in biological fluids through bioelectrocatalytic reactions 128 , 137 , 138 ; for example, using ascorbate in tears as the fuel, a self-powered contact lens can monitor tear glucose levels 139 . Similarly, a self-powered wireless sensing system based on glucose and lactate biofuel cells can monitor sweat glucose and lactate levels 140 . If a single power source is insufficient to power the device, a microgrid system incorporating biofuel cells, triboelectric generators and supercapacitors can provide higher power output 141 .
Long-term wearable electrochemical biosensors can be designed with flexible electrode materials (for example, metals, conductive polymers and low-dimensional materials) that resist mechanical deformation (for example, strain and bending) and that can be self-healing 142 . In addition, flexible, printed circuit boards that contain full-featured microcontrollers and other components, such as communication modules, can be designed by commercial software, such as the Altium Designer, and fabricated by commercially printed circuit board manufacturers 143 . Wireless information communication technologies, such as Bluetooth 57 , 144 and near-field communication 145 , 146 , have low power consumption and acceptable communication distance, allowing sensing devices to communicate with remote electronic systems such as smartphones, which can analyze, display and store data (Fig. 3b ).
However, the performance of wearable biosensors is limited by variations in connectivity and impedances caused by human physical activities that can lead to detection errors. Signal processing and calibration algorithms can be applied to correct for such artefacts 147 ; for example, electrochemical signals that are affected by pH, temperature and flow rate can be calibrated by a multiplexed sensing strategy using lookup tables for real-time and automated calibration 57 . To reduce signal variation, an accelerometer can further be integrated and the signal can be filtered using short-time fast Fourier transform. More advanced frequency-domain algorithms, such as the wavelet-transform projection, can be employed to decouple motions from the electrochemical measurement 148 . Furthermore, the relative change in electrochemical signal (for example, Nernstian shift) can be used instead of the absolute signal value to decrease measurement errors 149 .
Integration into implantable devices
Finger-prick blood tests using portable electrochemical devices are usually highly accurate but require frequent, invasive sample collection 150 . Wearable electrochemical biosensing is non-invasive but suffers from low analytical accuracy, which is a particular concern in diagnostic applications 151 , 152 . Alternatively, implantable electrochemical biosensors combine the high accuracy of invasive finger-prick tests and the long-term monitoring capability of non-invasive wearable analysis 153 , 154 . Implantable electrochemical biosensors have been particularly explored for continuous glucose monitoring and in vivo monitoring of biomarkers, such as neurochemicals, in the brain 155 , 156 , 157 , 158 (Fig. 4 ).
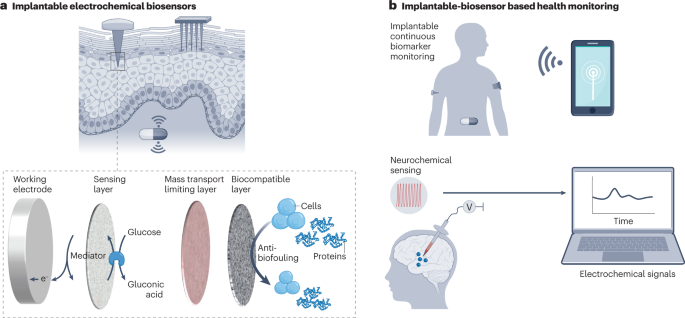
a , Microneedle-based implantable electrochemical biosensors for the monitoring of analytes in interstitial fluid. The working electrode is modified with multiple functional layers, including an inner sensing layer consisting of a redox polymer and an enzyme, a mass transport-limiting layer to improve stability, and an outer biocompatible layer to prevent fouling of the sensor. b , Implantable electrochemical biosensors allow continuous glucose monitoring and in vivo detection of neurochemicals in the brain.
In electrochemical biosensors, the detection reaction occurs on the surface of the electrodes and, thus, such sensors can easily be integrated with circuitry and incorporated into a small capsule for implantation 159 , 160 . Implantable electrochemical biosensors (for example, subcutaneous or intravascular) can provide dynamic information on glucose levels to guide therapy adjustments 157 , 161 , 162 , 163 . Similarly, spatiotemporal electrochemical sensing of neurochemicals, such as dopamine and acetylcholine, in the brain can indicate neuronal activity 164 , 165 , 166 .
Most implantable electrodes are made of Au, Pt and Ir, which are electrochemically stable and, in principle, biocompatible 155 , 167 , 168 . However, as foreign bodies, implantable devices are subject to biofouling and the foreign body response, compromising their analytical performance 169 . Therefore, the electrode has to be coated with multiple functional layers, including an inner sensing layer consisting of redox polymer and enzyme, a middle layer to improve stability, and an outer biocompatible layer to prevent fouling of the sensor 170 , 171 (Fig. 4a ). For example, NO-releasing polymer coatings can improve the biocompatibility of implantable biosensors (for example, intravascular sensors) because the endogenous gas molecule NO inhibits platelet adhesion and activation, inflammatory responses, and bacterial growth 148 , 172 . In addition, implantable devices need to be sterilized; thus, the coating layers need to withstand sterilization treatments such as irradiation 173 .
The mechanical mismatch between soft tissues and implantable electrodes may lead to inflammatory responses and/or device failure. Therefore, implantable electrodes should be soft and stretchable to seamlessly interface with soft tissues. For example, a soft implantable neurotransmitter sensor can monitor the dynamics of monoamine in the brain and gut of mice 174 ; here, the soft, elastic and thin electrode is fabricated by embedding laser-induced graphene nanofibres in an elastomer matrix, minimizing damage to intestinal tissue and not disturbing the peristaltic movement of the gastrointestinal tract.
Implantable biosensors typically remain in the body for long time periods, which requires an adequate power supply 175 with high volumetric energy density (that is, the energy stored per unit of volume) owing to the constraint of the device size 152 , 155 . Batteries have high energy densities but require periodic replacement, which may risk infection and additional costs 176 . Alternatively, implantable electrochemical biosensors could be made self-powered using piezoelectric materials, triboelectric materials or fuel cells 156 , 177 , 178 , 179 , 180 . In addition, near-field communication may enable wireless power generation and data transmission 159 , 181 .
Alternative to implantable devices that typically require surgery, partially implantable electrochemical biosensors have been commercialized (for example, Freestyle Libre from Abbott and G6 CGM system from Dexcom) 154 , 182 (Supplementary Table 1 ). Such partially implantable biosensors only require subcutaneous insertion of a small probe (for example, a flexible needle) or a probe array, leaving most components, including the power source, readout circuitry and wireless communication modules, on the surface of the skin 151 , 162 . For example, minimally invasive biosensors for glucose detection allow continuous glucose monitoring for about 2 weeks and can then be replaced by the patient 161 , 183 . However, these glucose biosensors are limited to single analyte analysis and may cause discomfort owing to the long needles (5–11 mm) that need to be inserted to access interstitial fluid. To achieve multiplexed analysis of biomarkers and discomfort-free operation, an integrated microneedle array can be applied that allows continuous monitoring of two analytes (for example, lactate and glucose, or alcohol and glucose) in interstitial fluid 184 . This device integrates reusable electronics to acquire and wirelessly transmit the electrochemical signals to a smartphone for data analysis and visualization.
Electrochemical biosensors are powerful tools to quantitatively analyze biochemical analytes in body fluids, providing digital data of dynamic physiological processes for fundamental research and health-care applications. The integration of electrochemical biosensors in portable, wearable and implantable devices enables decentralized POC detection 185 , 186 , 187 , which has the potential to revolutionize diagnostics and health management 188 , particularly in low-resource settings (Box 1 ). Batch fabrication and integration of disposable, flexible and multi-electrode electrochemical biosensors with different substrates, including plastics, flexible films, textiles and paper, can be achieved by printing (for example, screen 28 , 29 , 30 , inkjet 122 , roll-to-roll 189 and transfer 190 printing) and microfabrication (for example, photolithography 57 , evaporation 124 , electron beam evaporation 114 , 119 and laser cutter 121 ); however, engineering challenges remain to be addressed for integrated electrochemical biosensors to make a real impact in POC diagnostics; for example, signal transduction, conditioning (amplification and filtering), processing and wireless transmission need to be improved 57 ; all functional controllers and modules should be integrated on one circuit board; packaging of soft electronics and chipsets needs to be optimized; and microminiaturization, networking and intellectualization of devices needs to be realized 191 (Box 2 ).
Beyond glucose sensing, electrochemical biosensing devices could also allow the POC detection of proteins, nucleic acids, viruses and cells; however, this will require automated multistep and multisolution technology. Digital microfluidics may enable full-automatic on-chip measurements but requires high-precision instruments, limiting its applications in low-resource settings. Therefore, simple, cheap, robust and stable microfluidic systems need to be developed, for example, using paper or hydrophilic and hydrophobic polymers, which can be folded and/or printed into low-cost, disposable devices. Importantly, electrochemical biosensors need to be engineered that achieve one-step biosensing to avoid complex handling processes. In addition, although amperometric, voltammetric, potentiometric and electrochemiluminescent biosensor devices have been commercialized, these are often invasive portable devices rather than non-invasive wearable and implantable devices, in particular, OECT, photoelectrochemical and electrochemiluminescent bioimaging biosensors are still at an early stage. Thus, electrochemical sensors need to be developed according to their specific properties; for example, OECT sensors can be developed for miniaturized wearable devices and photoelectrochemical sensors can be developed for miniaturized composite implantable devices (Table 1 ).
Smartphones, 5G communication and cloud computing will allow the digitalization of health-related information obtained by integrated electrochemical biosensors. For example, physical sensors connected and/or integrated into smartphones, watches or wristbands allow the daily monitoring of vital signs such as heart rate, electrocardiogram and electroencephalogram. Similarly, electrochemical biosensors can be integrated into wearable devices for the non-invasive monitoring of specific analytes in body fluids related to health management.
Engineering efforts are often dedicated to improving the sensitivity, selectivity and multiplex capability of electrochemical biosensors, making these devices increasingly complex and prone to failure. However, detection sensitivity and selectivity mainly depend on the recognition reaction at the delicate electrolyte–electrode interface, which is affected by a range of factors, such as the friction between electrodes and tissues, and the dynamic change of pH, flow rate and temperature of the body fluid, particularly in wearable devices. Therefore, more robust and maintenance-free electrochemical biosensors need to be designed that allow long-term health monitoring; for example, enzyme-based sensing chemistry can be replaced by nanomaterial-based catalytic sensing chemistry, which is less influenced by environmental conditions such as temperature, pH and ionic strength. In addition, the accuracy and reliability of electrochemical biosensors could be improved by implementing biosensor arrays that enable multiple detections in different environmental conditions. Such arrays can be built using all-solid-state electrodes, which can easily be integrated into printed circuit boards. The convoluted signals measured by the array can then be deconvoluted using algorithms, such as Fourier and wavelet transformation, to achieve simultaneous, multiplex detection. Furthermore, sensing accuracy could be improved by applying techniques commonly used in electrocardiograms, electromyograms and magnetic resonance imaging; for example, compressed sensing, which enables sub-Nyquist processing of sparse signals 192 , 193 .
Commercialization and broad applicability of integrated electrochemical biosensors will require concerted efforts in refining sensing techniques and flexible materials and in consolidating electronics, wireless electronics, data processing and data mining.
Box 1 Low-resource considerations
To achieve point-of-care analysis of health-related molecules in low-resource settings, electrochemical biosensing devices need to be portable, cheap, simple to operate and provide rapid readout and data analysis. In addition, storage and long-term stability should be considered. For example, electrochemical biosensors can be designed as disposable test strips and results can be detected with a handheld reader. The test strips (for example, blood glucose test strip) often have a shelf life of several months at room temperature in dry conditions, allowing transportation and storage without requiring a cold chain. Such a simple design is also compatible with large-scale industrial manufacturing workflows, which lowers the cost. Devices designed as test strips provide accurate and rapid sample-to-answer detection for point-of-care applications without requiring trained personnel. In addition, integration of electrochemical biosensors in smartphones, watches and wristbands enables at-home measurement of biophysiological molecules for health monitoring and disease diagnosis.
Box 2 Translational considerations
The clinical translation of electrochemical biosensors for point-of-care diagnostic devices requires the establishment of diagnostic criteria for the evaluation of test results in different sample types. For example, diagnostic criteria for glucose tests have been well-established for blood samples; however, diagnostic criteria for other body fluids, such as sweat, saliva and tears, are more difficult to define. In addition, compared with blood samples, these biofluid samples may be affected by sampling location (for example, saliva in different positions in the mouth, sweat from different sweat glands) and by the environment (for example, before and after exercise or water drinking). Therefore, the comparison of test results and validation of test criteria remains challenging. Thus, the translational process of biosensing devices for non-blood samples may differ from that of blood samples, requiring the standardization of body fluid sampling and additional sensing units to monitor the dynamic change in pH, temperature and flow rate of the body fluid for calibration. In addition, commercialization of the blood glucose meter was originally based on blood glucose measurements in hospital settings, outlining the criteria for the design of the device; by contrast, new electrochemical biosensor-based devices intended for other body fluids may not be based on experience in hospital settings but may instead be tested and validated as consumer devices for early health warning and health management in lifestyle and fitness.
The translation of electrochemical biosensors will further depend on their ability to perform full-automatic electrochemical biosensing of affinity analytes. This can be achieved by the integration of test strips with automatic microfluidic systems. However, microfluidic systems are typically fabricated using high-cost materials and microfabrication technologies (for example, soft lithography) 125 , 194 , 195 , 196 . Cheap but robust and stable microfluidic systems (for example, paper-based microfluidics) should thus be further developed to promote the application of biosensor devices in health monitoring.
Clark, L. C. Jr & Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. NY Acad. Sci. 102 , 29–45 (1962).
Article Google Scholar
Lambrianou, A., Demin, S. & Hall, E. A. H. Biosensing for the 21st Century (eds Renneberg, R., & Lisdat, F.) 65–95 (Springer, 2007).
Labib, M., Sargent, E. H. & Kelley, S. O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 116 , 9001–9090 (2016). This review introduces electrochemical sensors fabricated with different recognition elements for the detection of small molecules, nucleic acids and proteins.
Wu, J., Fu, Z. F., Yan, F. & Ju, H. X. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trends Anal. Chem. 26 , 679–688 (2007).
Minteer, S. D. Advances in electroanalytical chemistry. J. Am. Chem. Soc. 140 , 2701–2703 (2018).
Zhang, J. J., Xiang, Y., Wang, M., Basu, A. & Lu, Y. Dose-dependent response of personal glucose meters to nicotinamide coenzymes: applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew. Chem. Int. Ed. 128 , 742–746 (2016).
Das, A., Cui, X. K., Chivukula, V. & Iyer, S. S. Detection of enzymes, viruses, and bacteria using glucose meters. Anal. Chem. 90 , 11589–11598 (2018).
Gong, S. H., Li, J. J., Pan, W., Li, N. & Tang, B. Duplex-specific nuclease-assisted CRISPR-Cas12a strategy for microRNA detection using a personal glucose meter. Anal. Chem. 93 , 10719–10726 (2021).
Liu, R., Hu, Y. S., He, Y., Lan, T. & Zhang, J. J. Translating daily COVID-19 screening into a simple glucose test: a proof of concept study. Chem. Sci. 12 , 9022–9030 (2021).
Cao, Y. Z. et al. Portable and sensitive detection of non-glucose target by enzyme-encapsulated metal-organic-framework using personal glucose meter. Biosens. Bioelectron. 198 , 113819 (2022).
Lubin, A. A. & Plaxco, K. W. Folding-based electrochemical biosensors: the case for responsive nucleic acid architectures. Acc. Chem. Res. 43 , 496–505 (2010). This review of folding-based electrochemical biosensors research opens up ways to fabricate one-step response affinity biosensors for nucleic acid and protein targets.
Zhang, H. Q., Li, F., Dever, B., Li, X. F. & Le, X. C. DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev. 113 , 2812–2841 (2013). This review introduces proximity binding-based affinity sensing mechanisms for one-step, homogenous detection of nucleic acid and protein targets.
Liu, B. W., Du, D., Hua, X., Yu, X. Y. & Lin, Y. H. Paper-based electrochemical biosensors: from test strips to paper-based microfluidics. Electroanalysis 26 , 1214–1223 (2014).
Sassa, F., Biswas, G. C. & Suzuki, H. Microfabricated electrochemical sensing devices. Lab Chip 20 , 1358–1389 (2020).
Noviana, E., McCord, C. P., Clark, K. M., Jang, I. & Henry, C. S. Electrochemical paper-based devices: sensing approaches and progress toward practical applications. Lab Chip 20 , 9–34 (2020).
Lei, J. P. & Ju, H. X. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 41 , 2122–2134 (2012). This review introduces nanomaterial-based signal amplification strategies, including functionalization methods for nanoprobes and surfaces, the functions of nanomaterials and their applications for ultrasensitive biosensing of biomolecules.
Ju, H. X. Biosensors: signal amplification for highly sensitive bioanalysis based on biosensors or biochips. J. Biochips Tissue Chips 2 , e114 (2012).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotech. 37 , 407–419 (2019).
Yu, Y., Nyein, H. Y. Y., Gao, W. & Javey, A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 32 , 1902083 (2020).
Xu, C. H., Yang, Y. R. & Gao, W. Skin-interfaced sensors in digital medicine: from materials to applications. Matter 2 , 1414–1445 (2020).
Song, Y., Min, J. H. & Gao, W. Wearable and implantable electronics: moving toward precision therapy. ACS Nano 13 , 12280–12286 (2019).
Hussein, H. A. et al. SARS-CoV-2-impedimetric biosensor: virus-imprinted chips for early and rapid diagnosis. ACS Sens. 6 , 4098–4107 (2021).
Zeng, J. et al. An impedimetric biosensor for COVID-19 serology test and modification of sensor performance via dielectrophoresis force. Biosen. Bioelectron. 213 , 114476 (2022).
Karyakin, A. A., Gitelmacher, O. V. & Karyakina, E. E. Prussian blue-based first-generation biosensor: a sensitive amperometric electrode for glucose. Anal. Chem. 67 , 2419–2423 (1995).
Ju, H. X., Zhang, X. J. & Wang, J. NanoBiosensing: Principles, Development and Application 85–102 (Springer, 2011).
Kim, S. K. et al. Bimetallic nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens. Adv. Mater. 34 , 2110536 (2022).
Huang, Y. Z., Han, Y. K., Sun, J. Y., Zhang, Y. & Han, L. Dual nanocatalysts co-decorated three-dimensional, laser-induced graphene hybrid nanomaterials integrated with a smartphone portable electrochemical system for point-of-care non-enzymatic glucose diagnosis. Mater. Today Chem. 24 , 100895 (2022).
Wu, J. et al. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 22 , 102–108 (2006).
Wu, J., Yan, F., Tang, J. H., Zhai, C. & Ju, H. X. A disposable multianalyte electrochemical immunosensor array for automated simultaneous determination of tumor markers. Clin. Chem. 53 , 1495–1502 (2007).
Wu, J. et al. Disposable reagentless electrochemical immunosensor array based on a biopolymer/sol-gel membrane for simultaneous measurement of several tumor markers. Clin. Chem. 54 , 1481–1488 (2008).
Fernandez-la-Villa, A., Pozo-Ayuso, D. F. & Castano-Alvarez, M. Microfluidics and electrochemistry: an emerging tandem for next-generation analytical microsystems. Curr. Opin. Electrochem. 15 , 175–185 (2019).
Kikkeri, K., Wu, D. & Voldman, J. A sample-to-answer electrochemical biosensor system for biomarker detection. Lab Chip 22 , 100–107 (2022).
Koklu, A. et al. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-beta detection. ACS Nano 15 , 8130–8141 (2021).
Sun, S. et al. Multifunctional self-driven origami paper-based integrated microfluidic chip to detect CRP and PAB in whole blood. Biosens. Bioelectron. 208 , 114225 (2022).
Feng, D. Z. et al. DNA tetrahedron-mediated immune-sandwich assay for rapid and sensitive detection of PSA through a microfluidic electrochemical detection system. Microsyst. Nanoeng. 7 , 33 (2021).
Lee, G. H. et al. Single microfluidic electrochemical sensor system for simultaneous multi-pulmonary hypertension biomarker analyses. Sci. Rep. 7 , 7545 (2017).
Pursey, J. P., Chen, Y., Stulz, E., Park, M. K. & Kongsuphol, P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuators B Chem. 251 , 34–39 (2017).
Fragoso, A. et al. Integrated microfluidic platform for the electrochemical detection of breast cancer markers in patient serum samples. Lab Chip 11 , 625–631 (2011).
Zhao, Y. X., Chen, F., Li, Q., Wang, L. H. & Fan, C. H. Isothermal amplification of nucleic acids. Chem. Rev. 115 , 12491–12545 (2015).
Simmel, F. C., Yurke, B. & Singh, H. R. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 119 , 6326–6369 (2019).
Dai, Y. F. et al. Exploring the trans-cleavage activity of CRISPR-Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. 58 , 17399–17405 (2019).
Zhang, D. C. et al. CRISPR/Cas12a-mediated interfacial cleaving of hairpin DNA reporter for electrochemical nucleic acid sensing. ACS Sens. 5 , 557–562 (2020).
Hu, J. M., Wang, T. Y., Kim, J., Shannon, C. & Easley, C. J. Quantitation of femtomolar protein levels via direct readout with the electrochemical proximity assay. J. Am. Chem. Soc. 134 , 7066–7072 (2012).
Hu, J. M. et al. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J. Am. Chem. Soc. 136 , 8467–8474 (2014).
Ren, K. W., Wu, J., Yan, F. & Ju, H. X. Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci. Rep. 4 , 4360 (2014).
Ren, K. W., Wu, J., Yan, F., Zhang, Y. & Ju, H. X. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 66 , 345–349 (2015).
Ren, K. W., Wu, J., Zhang, Y., Yan, F. & Ju, H. X. Proximity hybridization regulated DNA biogate for sensitive electrochemical immunoassay. Anal. Chem. 86 , 7494–7499 (2014).
Ren, K. W., Wu, J., Ju, H. X. & Yan, F. Target-driven triple-binder assembly of MNAzyme for amplified electrochemical immunosensing of protein biomarker. Anal. Chem. 87 , 1694–1700 (2015).
Zhu, J., Gan, H. Y., Wu, J. & Ju, H. X. Molecular machine powered surface programmatic chain reaction for highly sensitive electrochemical detection of protein. Anal. Chem. 90 , 5503–5508 (2018).
Man, Y. et al. An anchored monopodial DNA walker triggered by proximity hybridization for amplified amperometric biosensing of nucleic acid and protein. Anal. Chim. Acta 1107 , 48–54 (2020).
Karimi-Maleh, H. et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 184 , 113252 (2021).
Ding, J. W., Chen, Y., Wang, X. W. & Qin, W. Label-free and substrate-free potentiometric aptasensing using polycation-sensitive membrane electrodes. Anal. Chem. 84 , 2055–2061 (2012).
Nurlely, A. M., Heng, L. Y. & Tan, L. L. Potentiometric enzyme biosensor for rapid determination of formaldehyde based on succinimide-functionalized polyacrylate ion-selective membrane. Measurement 175 , 109112 (2021).
Özbek, O., Berkel, C., Isildak, Ö. & Isildak, I. Potentiometric urea biosensors. Clin. Chim. Acta 524 , 154–163 (2022).
Wu, J. et al. Potentiometric detection of DNA hybridization using enzyme-induced metallization and a silver ion selective electrode. Anal. Chem. 81 , 10007–10012 (2009).
Ding, J. W., Li, B. W., Chen, L. X. & Qin, W. A three-dimensional origami paper-based device for potentiometric biosensing. Angew. Chem. Int. Ed. 55 , 13033–13037 (2016).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529 , 509–514 (2016). This article demonstrates a mechanically flexible and fully integrated wearable system for sweat metabolites and electrolytes as well as for skin temperature monitoring.
Tang, H., Yan, F., Lin, P., Xu, J. B. & Chan, H. L. W. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 21 , 2264–2272 (2011).
Tang, H., Lin, P., Chan, H. L. W. & Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 26 , 4559–4563 (2011).
Mak, C. H. et al. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. J. Mater. Chem. C. 3 , 6532–6538 (2015).
Parlak, O., Keene, S. T., Marais, A., Curto, V. F. & Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 4 , eaar2904 (2018).
Lin, P., Luo, X. T., Hsing, I. M. & Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 23 , 4035–4040 (2011).
Fu, Y. et al. Highly sensitive detection of protein biomarkers with organic electrochemical transistors. Adv. Mater. 29 , 1703787 (2017).
Kim, D. J. et al. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 25 , 2477–2482 (2010).
He, R. X. et al. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem. 22 , 22072–22076 (2012).
Lin, B. P., Yan, F., Yu, J. J., Chan, H. L. W. & Yang, M. The application of organic electrochemical transistors in cell-based biosensors. Adv. Mater. 22 , 3655–3660 (2010).
Chen, L. Z. et al. Organic electrochemical transistors for the detection of cell surface glycans. ACS Appl. Mater. Interfaces 10 , 18470–18477 (2018).
Chen, L. Z., Wang, N. X., Wu, J., Yan, F. & Ju, H. X. Organic electrochemical transistor for sensing of sialic acid in serum samples. Anal. Chim. Acta 1128 , 231–237 (2020).
Chen, L. Z., Wu, J., Yan, F. & Ju, H. X. A facile strategy for quantitative sensing of glycans on cell surface using organic electrochemical transistors. Biosens. Bioelectron. 175 , 112878 (2021).
Chen, L. Z., Wu, J., Yan, F. & Ju, H. X. Monose-modified organic electrochemical transistors for cell surface glycan analysis via competitive recognition to enzyme-labeled lectin. Mikrochim. Acta 188 , 1–7 (2021).
Wang, N., Yang, A., Fu, Y., Li, Y. & Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 52 , 277–287 (2019).
Yang, A. N. et al. Fabric organic electrochemical transistors for biosensors. Adv. Mater. 30 , 1800051 (2018).
Zang, Y., Lei, J. P. & Ju, H. X. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens. Bioelectron. 96 , 8–16 (2017).
Ruan, Y. F. et al. An integrated photoelectrochemical nanotool for intracellular drug delivery and evaluation of treatment effect. Angew. Chem. Int. Ed. 133 , 25966–25969 (2021).
Hu, F. X., Miao, J. W., Guo, C. X., Yang, H. B. & Liu, B. Real-time photoelectrochemical quantification of hydrogen peroxide produced by living cells. Chem. Eng. J. 407 , 127203 (2021).
Ye, X. X. et al. FRET modulated signaling: a versatile strategy to construct photoelectrochemical microsensors for in vivo analysis. Angew. Chem. Int. Ed. 60 , 11774–11778 (2021).
Qi, H. L. & Zhang, C. X. Electrogenerated chemiluminescence biosensing. Anal. Chem. 92 , 524–534 (2020).
Chen, Y. H. & Ding, Z. F. Highly sensitive analysis strategies of microRNAs based on electrochemiluminescence. Curr. Opin. Electrochem. 32 , 100901 (2022).
Miao, W. J. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 108 , 2506–2553 (2008).
Delaney, J. L., Hogan, C. F., Tian, J. & Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 83 , 1300–1306 (2011).
Kerr, E. et al. Amplification-free electrochemiluminescence molecular beacon-based microRNA sensing using a mobile phone for detection. Sens. Actuators B Chem. 330 , 129261 (2021).
Deiss, F. et al. Multiplexed sandwich immunoassays using electrochemiluminescence imaging resolved at the single bead level. J. Am. Chem. Soc. 131 , 6088–6089 (2009).
Wang, N. N., Chen, L. Z., Chen, W. W. & Ju, H. X. Potential-and color-resolved electrochemiluminescence of polymer dots for array imaging of multiplex microRNAs. Anal. Chem. 93 , 5327–5333 (2021).
Ma, C., Cao, Y., Gou, X. D. & Zhu, J. J. Recent progress in electrochemiluminescence sensing and imaging. Anal. Chem. 92 , 431–454 (2020).
Wang, N. N. et al. Dual intramolecular electron transfer for in situ coreactant‐embedded electrochemiluminescence microimaging of membrane protein. Angew. Chem. Int. Ed. 60 , 197–201 (2021).
Wang, N. N. et al. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 201 , 113959 (2022).
Teymourian, H., Barfidokht, A. & Wang, J. Electrochemical glucose sensors in diabetes management: an updated review (2010-2020). Chem. Soc. Rev. 49 , 7671–7709 (2020).
Nagata, R., Yokoyama, K., Clark, S. A. & Karube, I. A glucose sensor fabricated by the screen printing technique. Biosens. Bioelectron. 10 , 261–267 (1995).
Wang, J. Electrochemical glucose biosensors. Chem. Rev. 108 , 814–825 (2008).
Tonyushkina, K. & Nichols, J. H. Glucose meters: a review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 3 , 971–980 (2009).
Karon, B. S., Boyd, J. C. & Klee, G. G. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin. Chem. 56 , 1091–1097 (2010).
Xiang, Y. & Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 3 , 697–703 (2011). This article demonstrates a method based on the target-induced release of invertase from a functional DNA–invertase conjugate to enable the quantification of non-glucose targets using a commercial personal glucose metre.
Zhang, J. & Lu, Y. Biocomputing for portable, resettable, and quantitative point-of-care diagnostics: making the glucose meter a logic-gate responsive device for measuring many clinically relevant targets. Angew. Chem. Int. Ed. 57 , 9702–9706 (2018).
Amalfitano, E. et al. A glucose meter interface for point-of-care gene circuit-based diagnostics. Nat. Commun. 12 , 724 (2021).
Zhang, X. et al. Electrochemical assay to detect influenza viruses and measure drug susceptibility. Angew. Chem. Int. Ed. 54 , 5929–5932 (2015).
Ahn, J. K., Kim, H. Y., Park, K. S. & Park, H. G. A personal glucose meter for label-free and washing-free biomolecular detection. Anal. Chem. 90 , 11340–11343 (2018).
Singh, N. K. et al. Hitting the diagnostic sweet spot: point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. Biosens. Bioelectron. 180 , 113111 (2021).
Rackus, D. G., Shamsi, M. H. & Wheeler, A. R. Electrochemistry, biosensors and microfluidics: a convergence of fields. Chem. Soc. Rev. 44 , 5320–5340 (2015).
de Campos, R. P. S. et al. “Plug-n-Play” sensing with digital microfluidics. Anal. Chem. 91 , 2506–2515 (2019).
Zhao, H. et al. Accessible detection of SARS-CoV-2 through molecular nanostructures and automated microfluidics. Biosens. Bioelectron. 194 , 113629 (2021).
Gong, M. M. & Sinton, D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 117 , 8447–8480 (2017).
Dungchai, W., Chailapakul, O. & Henry, C. S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 81 , 5821–5826 (2009).
Liu, H. & Crooks, R. M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc. 133 , 17564–17566 (2011).
Liu, H., Xiang, Y., Lu, Y. & Crooks, R. M. Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angew. Chem. Int. Ed. 51 , 6925–6928 (2012). This article reports a method to fabricate a self-powered origami paper analytical device with a simple digital multimetre for signal readout.
Chen, C. A. et al. An electricity- and instrument-free infectious disease sensor based on a 3D origami paper-based analytical device. Lab Chip 21 , 1908–1915 (2021).
Su, J. et al. Smartphone-based electrochemical biosensors for directly detecting serum-derived exosomes and monitoring their secretion. Anal. Chem. 94 , 3235–3244 (2022).
Guo, J. H. Smartphone-powered electrochemical biosensing dongle for emerging medical IoTs application. IEEE T. Ind. Inform. 14 , 2592–2597 (2018).
Ainla, A. et al. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 90 , 6240–6246 (2018).
Kim, J., Campbell, A. S., de Avila, B. E. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37 , 389–406 (2019). This review discusses the development of wearable biosensing systems for health-care monitoring, including their fundamental principles, prototypes and applications for different biological fluids.
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1 , 160–171 (2018).
Park, J. et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 4 , eaap9841 (2018).
Moyer, J., Wilson, D., Finkelshtein, I., Wong, B. & Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14 , 398–402 (2012).
Baker, L. B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6 , 211–259 (2019).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114 , 4625–4630 (2017).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9 , 031301 (2015).
Lu, Y., Jiang, K., Chen, D. & Shen, G. Z. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy 58 , 624–632 (2019).
Arakawa, T. et al. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 92 , 12201–12207 (2020).
Khalil, O. S. Noninvasive photonic-crystal material for sensing glucose in tears. Clin. Chem. 50 , 2236–2237 (2004).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7 , eabg9614 (2021).
Teymourian, H. et al. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 5 , 2679–2700 (2020).
Wang, M. Q. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6 , 1225–1235 (2022).
Yu, Y. et al. All-printed soft human-machine interface for robotic physicochemical sensing. Sci. Robot. 7 , eabn0495 (2022).
Quinton, P. M. Sweating and its disorders. Annu. Rev. Med. 34 , 429–452 (1983).
Nyein, H. Y. Y. et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 12 , 1823 (2021).
Choi, J., Kang, D., Han, S., Kim, S. B. & Rogers, J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6 , 1601355 (2017).
Lin, H. et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 11 , 4405 (2020).
Manjakkal, L., Yin, L., Nathan, A., Wang, J. & Dahiya, R. Energy autonomous sweat-based wearable systems. Adv. Mater. 33 , 2100899 (2021).
Chong, Y. W., Ismail, W., Ko, K. & Lee, C. Y. Energy harvesting for wearable devices: a review. IEEE Sens. J. 19 , 9047–9062 (2019).
Lou, Z., Wang, L. L. & Shen, G. Z. Recent advances in smart wearable sensing systems. Adv. Mater. Technol. 3 , 1800444 (2018).
Yang, P. et al. Monitoring the degree of comfort of shoes in-motion using triboelectric pressure sensors with an ultrawide detection range. ACS Nano 16 , 4654–4665 (2022).
Yang, J. H. et al. Effect of garment design on piezoelectricity harvesting from joint movement. Smart Mater. Struct. 25 , 035012 (2016).
Fu, K., Zhou, J., Wu, H. U. & Su, Z. Q. Fibrous self-powered sensor with high stretchability for physiological information monitoring. Nano Energy 88 , 106258 (2021).
Hao, Y. et al. Self-rebound cambered triboelectric nanogenerator array for self-powered sensing in kinematic analytics. ACS Nano 16 , 1271–1279 (2022).
Luo, X. X. et al. Tribovoltaic nanogenerators based on mxene-silicon heterojunctions for highly stable self-powered speed, displacement, tension, oscillation angle, and vibration sensors. Adv. Funct. Mater. 32 , 2113149 (2022).
Article MathSciNet Google Scholar
Song, Y. et al. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 6 , eaay9842 (2020).
Qin, Y. et al. Stretchable triboelectric self-powered sweat sensor fabricated from self-healing nanocellulose hydrogels. Adv. Funct. Mater. 32 , 2201846 (2022).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74 , 1061–1068 (2015).
Jia, W., Valdes-Ramirez, G., Bandodkar, A. J., Windmiller, J. R. & Wang, J. Epidermal biofuel cells: energy harvesting from human perspiration. Angew. Chem. Int. Ed. 52 , 7233–7236 (2013).
Falk, M., Andoralov, V., Silow, M., Toscano, M. D. & Shleev, S. Miniature biofuel cell as a potential power source for glucose-sensing contact lenses. Anal. Chem. 85 , 6342–6348 (2013).
Huang, X. C. et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 5 , 201–209 (2022).
Yin, L. et al. A self-sustainable wearable multi-modular E-textile bioenergy microgrid system. Nat. Commun. 12 , 1542 (2021).
Chen, Z., Xi, J., Huang, W. & Yuen, M. M. F. Stretchable conductive elastomer for wireless wearable communication applications. Sci. Rep. 7 , 10958 (2017).
Takei, K. et al. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 9 , 821–826 (2010).
Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 3 , 944–952 (2018).
Rose, D. P. et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 62 , 1457–1465 (2015).
Keum, D. H. et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6 , eaba3252 (2020).
Lukocius, R., Vaitkunas, M., Virbalis, J. A., Dosinas, A. & Vegys, A. Physiological parameters monitoring system for occupational safety. Elektron. Elektrotech. 20 , 57–60 (2014).
Lee, K. et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 4 , 148–158 (2020).
Bujes-Garrido, J. & Arcos-Martínez, M. J. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 240 , 224–228 (2017).
Vaddiraju, S., Burgess, D. J., Tomazos, I., Jain, F. C. & Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 4 , 1540–1562 (2010).
Zhang, J. et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv. Healthc. Mater. 10 , 2100194 (2021).
Rodrigues, D. et al. Skin-integrated wearable systems and implantable biosensors: a comprehensive review. Biosensors 10 , 79 (2020).
Bobrowski, T. & Schuhmann, W. Long-term implantable glucose biosensors. Curr. Opin. Electrochem. 10 , 112–119 (2018).
Li, P. et al. From diagnosis to treatment: recent advances in patient-friendly biosensors and implantable devices. ACS Nano 15 , 1960–2004 (2021).
Dalrymple, A. N. Implanted devices: the importance of both electrochemical performance and biological acceptance. Neural Regen. Res. 16 , 1188–1189 (2021).
Kotanen, C. N., Moussy, F. G., Carrara, S. & Guiseppi-Elie, A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 35 , 14–26 (2012).
Li, C. M., Dong, H., Cao, X., Luong, J. H. & Zhang, X. Implantable electrochemical sensors for biomedical and clinical applications: progress, problems, and future possibilities. Curr. Med. Chem. 14 , 937–951 (2007).
Wagner, J., Tennen, H. & Wolpert, H. Continuous glucose monitoring: a review for behavioral researchers. Psychosom. Med. 74 , 356–365 (2012).
Xu, J. et al. Implantable platinum nanotree microelectrode with a battery-free electrochemical patch for peritoneal carcinomatosis monitoring. Biosens. Bioelectron. 185 , 113265 (2021).
Singh, P. et al. Biomedical perspective of electrochemical nanobiosensor. Nanomicro Lett. 8 , 193–203 (2016).
Google Scholar
McGarraugh, G. The chemistry of commercial continuous glucose monitors. Diabetes Technol. Ther. 11 , S17–S24 (2009).
Gross, T. M. et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2 , 49–56 (2000).
Lee, H., Hong, Y. J., Baik, S., Hyeon, T. & Kim, D. H. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 7 , 1701150 (2018).
Clark, J. J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7 , 126–129 (2010).
Tavakolian-Ardakani, Z., Hosu, O., Cristea, C., Mazloum-Ardakani, M. & Marrazza, G. Latest trends in electrochemical sensors for neurotransmitters: a review. Sensors 19 , 2037 (2019).
Azzouz, A. et al. Nanomaterial-based electrochemical sensors for the detection of neurochemicals in biological matrices. Trends Anal. Chem. 110 , 15–34 (2019).
Lei, L. J. et al. Nonenzymatic electrochemical sensor for wearable interstitial fluid glucose monitoring. Electroanalysis 34 , 415–422 (2022).
Seaton, B. T. & Heien, M. L. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 413 , 6689–6701 (2021).
Zhang, D. H. et al. Dealing with the foreign-body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 31 , 2007226 (2021).
Wickramasinghe, Y., Yang, Y. & Spencer, S. A. Current problems and potential techniques in in vivo glucose monitoring. J. Fluoresc. 14 , 513–520 (2004).
Nichols, S. P., Koh, A., Storm, W. L., Shin, J. H. & Schoenfisch, M. H. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 113 , 2528–2549 (2013).
Malone-Povolny, M. J., Merricks, E. P., Wimsey, L. E., Nichols, T. C. & Schoenfisch, M. H. Long-term accurate continuous glucose biosensors via extended nitric oxide release. ACS Sens. 4 , 3257–3264 (2019).
Hetrick, E. M. & Schoenfisch, M. H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35 , 780–789 (2006).
Li, J. X. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606 , 94–101 (2022). This article introduces a tissue-mimicking, stretchable, neurotransmitter sensor fabricated by laser patterning and demonstrates its application for in vivo real-time, multichannel and multiplexed monoamine sensing in the mouse brain.
Ben Amar, A., Kouki, A. B. & Cao, H. Power approaches for implantable medical devices. Sensors 15 , 28889–28914 (2015).
Rebelo, R., Barbosa, A. I., Correlo, V. M. & Reis, R. L. An outlook on implantable biosensors for personalized medicine. Engineering 7 , 1696–1699 (2021).
Yang, Y., Wei, X. J. & Liu, J. Suitability of a thermoelectric power generator for implantable medical electronic devices. J. Phys. D Appl. Phys. 40 , 5790–5800 (2007).
Hwang, G. T. et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv. Mater. 26 , 4880–4887 (2014).
Zheng, Q. et al. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 26 , 5851–5856 (2014).
Simons, P., Schenk, S. A., Gysel, M. A., Olbrich, L. F. & Rupp, J. L. M. A ceramic-electrolyte glucose fuel cell for implantable electronics. Adv. Mater. 34 , 2109075 (2022).
Scholten, K. & Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 544 , 319–334 (2018).
Green, R. A., Lovell, N. H., Wallace, G. G. & Poole-Warren, L. A. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials 29 , 3393–3399 (2008).
Kutner, N., Kunduru, K. R., Rizik, L. & Farah, S. Recent advances for improving functionality, biocompatibility, and longevity of implantable medical devices and deliverable drug delivery systems. Adv. Funct. Mater. 31 , 2010929 (2021).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6 , 1214–1224 (2022). This article reports the fabrication of a fully integrated prototype of a wearable sensor patch-based microneedle array and introduces its application for the wireless and continuous real-time sensing of two metabolites in the interstitial fluid.
Hsieh, K., Ferguson, B. S., Eisenstein, M., Plaxco, K. W. & Soh, H. T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 48 , 911–920 (2015).
Zhang, J., Lan, T. & Lu, Y. Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. Trends Anal. Chem. 124 , 115782 (2020).
Loncaric, C., Tang, Y. T., Ho, C., Parameswaran, M. A. & Yu, H. Z. A USB-based electrochemical biosensor prototype for point-of-care diagnosis. Sens. Actuators B Chem. 161 , 908–913 (2012).
Tu, J. B., Torrente-Rodriguez, R. M., Wang, M. Q. & Gao, W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. 30 , 1906713 (2020).
Bariya, M. et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 12 , 6978–6987 (2018). This article reports batch fabrication of flexible and robust electrode arrays by roll-to-roll gravure printing for a range of electrochemical sensing applications.
Tian, L. et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 3 , 194–205 (2019). This article reports materials, device structures, handing and mounting methods, and manufacturing approaches for the fabrication of large-area epidermal electronic interfaces.
Gordon, W. J. & Stern, A. D. Challenges and opportunities in software-driven medical devices. Nat. Biomed. Eng. 3 , 493–497 (2019).
Liu, H. & Zhao, C. Wearable electrochemical sensors for noninvasive monitoring of health-a perspective. Curr. Opin. Electrochem. 23 , 42–46 (2020).
Dixon, A. M., Allstot, E. G., Gangopadhyay, D. & Allstot, D. J. Compressed sensing system considerations for ECG and EMG wireless biosensors. IEEE Trans. Biomed. Circuits Syst. 6 , 156–166 (2012).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl Med. 8 , 366ra165 (2016).
Kamei, K. et al. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices 17 , 36 (2015).
Martin, A. et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2 , 1860–1868 (2017).
Download references
Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (21827812, 21890741), the Science and Technology Project of Nanjing City (202110023) and the Independent Research Foundation from the State Key Laboratory of Analytical Chemistry for Life Science (5431ZZXM2006) for start-up supply. The authors are also grateful to Y. Lu in Fasteur Biotechnology for market information, to Q. Yu, Y.C. Chen and L.J. Lei for helpful comments.
Author information
These authors contributed equally: Jie Wu, Hong Liu.
Authors and Affiliations
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, China
Jie Wu, Weiwei Chen & Huangxian Ju
State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing, China
Hong Liu & Biao Ma
School of Geographic and Biologic Information, Nanjing University of Posts and Telecommunications, Nanjing, China
Weiwei Chen
You can also search for this author in PubMed Google Scholar
Contributions
J.W., H.L. and H.X.J. arranged the sections of the Review. J.W., W.W.C. and H.X.J. wrote the introduction and the section on electrochemical biosensing of disease biomarkers, and H.L., B.M and H.X.J. wrote the sections on portable electrochemical biosensing devices, integration into wearable devices and integration into implantable devices. All authors discussed the outlook section and display items.
Corresponding author
Correspondence to Huangxian Ju .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Bioengineering thanks Onur Parlak, Chung Chiun Liu, and Susana Campuzano for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Wu, J., Liu, H., Chen, W. et al. Device integration of electrochemical biosensors. Nat Rev Bioeng 1 , 346–360 (2023). https://doi.org/10.1038/s44222-023-00032-w
Download citation
Accepted : 23 January 2023
Published : 24 February 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s44222-023-00032-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Skin-inspired soft bioelectronic materials, devices and systems.
- Chuanzhen Zhao
Nature Reviews Bioengineering (2024)
Wide-range and high-accuracy wireless sensor with self-humidity compensation for real-time ammonia monitoring
- Jianhua Yang
Nature Communications (2024)
Development of an electrochemical sensor based on aniline, N-phenylglycine, graphene oxide and p-tertbutyl-calix-[4]-arene for trace detection of uranium ion from water
- Kusumita Dutta
- Siddhartha Panda
Journal of Applied Electrochemistry (2024)
Gold nanoparticles-based biosensors: pioneering solutions for bacterial and viral pathogen detection—a comprehensive review
- Vishakha Suryakant Parkhe
- Arpita Pandey Tiwari
World Journal of Microbiology and Biotechnology (2024)
An interfacial robust and entire self-healing ionogel-elastomer hybrid for elastic electronics enables discretionary assembly and reconfiguration
- Zhengwei You
Science China Chemistry (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Recent advances in electrochemical biosensors: applications, challenges, and future scope.

1. Introduction
2. types of biosensors, 2.1. catalytic biosensors, 2.2. affinity biosensors, 3. electrochemical biosensors, 3.1. amperometric biosensor, 3.2. voltammetric methods, 3.3. impedimetric biosensor, 3.4. potentiometric biosensors, 4. applications of electrochemical biosensors, 4.1. food industry, 4.2. medical sciences, 4.3. defence, 4.4. metabolic engineering and plant biology, 5. machine learning for biosensors, 5.1. improvement in biosensor by ml, 5.2. various algorithms in ml, 5.3. ml data analysis, 5.3.1. support vector machine (svm), 5.3.2. feedforward artificial neural networks (ann), 5.3.3. convolutional neural network (cnn), 5.3.4. recurrent neural networks (rnn), 6. challenges and solution, 6.1. challenges.
- The LOD determines the lowest limit of the analyte that can be detected by a sensor and ideal biosensors must have a very low value of LOD.
- The reproducibility of sensors is very important when it comes to their fabrication and marketing. The results obtained for a particular sensor must be reproducible to all the similar sensors produced, as testing each sensor will not be possible.
- Finally, the most important characteristic of a sensor is its application to real samples. If a sensor is not effective in testing a real sample, it cannot be used in the diagnosis. The real samples that are mostly used for electrochemical biosensors are saliva, blood, urine, sweat, body fluid, tears, etc. The real sample collection is itself a challenge; some factors need to be considered for collecting a real sample for detection.
- The matrix effect in case of electrochemical sensors interferes with the sensor performance. To avoid this matrix effect, the real sample needs to be diluted, but extra dilution may cause deviation from reality. An ideal electrochemical biosensor should sense a real sample without requiring any processing and dilution. Similarly, the samples collected via saliva need dilution before sensing and the pH variation is the problem with the urine samples affecting the peak position. The tear samples due to less complexity have been used for diabetes detection, but the pH variation is again a challenge. Moreover, the concentration of the analytes in the tears produced from irritation and emotion may differ from each other. Moreover, the real samples contain species like protein, fats, etc. that may get adsorbed on the sensor surface and impact the sensitivity and reproducibility of an electrochemical biosensor. The researchers are looking for advanced new materials and techniques (active and passive methods) to address this issue. In the active method, shear forces are produced that prevent the adhesion of the extra species on the sensor surface, whereas in passive methods, polymers are used to make the surface hydrophilic, thus preventing proteins from adsorption. The biosensors developed must be stable under extreme environmental conditions and hence, the stability of the electrochemical biosensors is very important.
6.2. Solutions
- Using nanomaterials might address the stability issue in some cases, but some nanomaterials seem to aggregate and reduce stability.
- The miniaturization of the electrochemical biosensors and using cheap materials in their fabrication is another step that needs to be taken in making them cheap.
- Micro-nano fabrication techniques are effective in reducing the size of the electrochemical biosensor. The smaller biosensors would be easy to use and dispose of, can be transported easily, and their application in extreme conditions would involve fewer efforts.
- The electrochemical biosensors have mostly been confined to the research labs. There needs to be a collaboration between clinics, hospitals, and research labs so that they can be tested in real-life circumstances, which will help in evaluating their performance. Multidisciplinary approach is important for further widespread use and commercialization of biosensors.
- On a global scale, bacterial diseases are responsible for the greatest number of deaths and illnesses. The electrochemical biosensors can prove effective in sensing these bacterial infections at early stages. These biosensors would also be very useful in detecting new pathogens in the water sources. However, huge efforts on technical and scientific ground will be required to make them more viable. The designing and fabrication process needs to be made more cost-effective. Moreover, the enzymatic electrochemical biosensors are used commonly in the research, but their stability and modification remain a concern. Another challenge is the storage of enzymes.
- The integration of electrochemical biosensors with POC devices would be a great initiative for application in clinics. Such biosensors would not be affected by the interference species and can detect any concentration of the analyte. In addition to this, nano technology will help in improving the LOD and sensitivity of the electrochemical biosensors.
7. Future Outlook
8. conclusions, author contributions, institutional review board statement, informed consent statement, conflicts of interest.
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999 , 71 , 2333–2348. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Khosla, A. Micropatternable Multifunctional Nanocomposite Polymers for Flexible Soft MEMS Applications. Ph.D. Thesis, Applied Science: School of Engineering Science, Simon Fraser University, Burnaby, BC, Canada, 2011. [ Google Scholar ]
- Ahmad, R.; Khan, M.; Tripathy, N.; Khan, M.I.R.; Khosla, A. Hydrothermally synthesiz0ed nickel oxide nanosheets for non-enzymatic electrochemical glucose detection. J. Electrochem. Soc. 2020 , 167 , 107504. [ Google Scholar ] [ CrossRef ]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.; Khosla, A.; Arya, S. Recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 2021 , 168 , 027505. [ Google Scholar ] [ CrossRef ]
- Chullasat, K.; Kanatharana, P.; Limbut, W.; Numnuam, A.; Thavarungkul, P. Ultra trace analysis of small molecule by label-free impedi-metric immunosensor using multilayer modified electrode. Biosens. Bioelectron. 2011 , 26 , 4571–4578. [ Google Scholar ] [ CrossRef ]
- Canbaz, M.Ç.; Şimşek, C.S.; Sezgintürk, M.K. Electrochemical biosensor based on self-assembled monolayersmodifi ed with gold nanoparticles for detection of HER-3. Anal Chim. Acta. 2014 , 814 , 31–38. [ Google Scholar ] [ CrossRef ]
- Kim, Y.H.; Park, J.S.; Jung, H.I. An impedimetric biosensor for real-time monitoring of bacterial growth in a microbial fermentor. Sens. Actuators B Chem. 2009 , 138 , 270–277. [ Google Scholar ] [ CrossRef ]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.S.; Hahn, Y.B. Recent advances in nanowires-based field-effect transistors for biological sensor applications. Biosens. Bioelectron. 2018 , 100 , 312–325. [ Google Scholar ] [ CrossRef ]
- Kumar, S.; Pavelyev, V.; Tripathi, N.; Platonov, V.; Sharma, P.; Ahmad, R.; Mishra, P.; Khosla, A. Review—Recent Advances in the Development of Carbon Nanotubes Based Flexible Sensors. J. Electrochem. Soc. 2020 , 167 , 047506. [ Google Scholar ] [ CrossRef ]
- Sheng, Y.; Zhang, T.; Zhang, S.; Johnston, M.; Zheng, X.; Shan, Y.; Liu, T.; Huang, Z.; Qian, F.; Xie, Z. A CRISPR/Cas13a-powered catalytic electrochemical biosensor for successive and highly sensitive RNA diagnostics. Biosens. Bioelectron. 2021 , 178 , 113027. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Srisomwat, C.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Pop-up paper electrochemical device for label-free hepatitis B virus DNA detection. Sens. Actuators B Chem. 2020 , 316 , 128077. [ Google Scholar ] [ CrossRef ]
- Carceller, J.M.; Mifsud, M.; Climent, M.J.; Iborra, S.; Corma, A. Production of chiral alcohols from racemic mixtures by integrated heterogeneous chemoenzymatic catalysis in fixed bed continuous operation. Green Chem. 2020 , 22 , 2767–2777. [ Google Scholar ] [ CrossRef ]
- Asefpour Vakilian, K.; Massah, J. A portable nitrate biosensing device using electrochemistry and spectroscopy. IEEE Sens. J. 2018 , 18 , 3080–3089. [ Google Scholar ] [ CrossRef ]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020 , 26 , 52. [ Google Scholar ] [ CrossRef ]
- Moon, G.; Choi, J.-R.; Lee, C.; Oh, Y.; Kim, K.; Kim, D. Machine learning-based design of meta-plasmonic biosensors with negative index metamaterials. Biosens. Bioelectron. 2020 , 164 , 112335. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Gonzalez-Macia, L.; Killard, A.J. Cholesterol biosensor based on inkjet-printed Prussian blue nanoparticle-modified screen-printed electrodes. Sens. Actuators B Chem. 2015 , 221 , 187–190. [ Google Scholar ] [ CrossRef ]
- Sannini, A.; Albanese, D.; Malvano, F.; Crescitelli, A.; Di Matteo, M. An amperometric biosensor for the determination of lactic acid during malolactic fermentation. Chem. Eng. Trans. 2015 , 44 , 283–288. [ Google Scholar ]
- Boffi, A.; Favero, G.; Federico, R.; Macone, A.; Antiochia, R.; Tortolini, C.B.; Sanzó, G.; Mazzei, F. Amine oxidase-based biosensors for spermine and spermidine determination. Anal. Bioanal. Chem. 2015 , 407 , 1131–1137. [ Google Scholar ] [ CrossRef ]
- Ciriello, R.; Cataldi, T.R.I.; Crispo, F.; Guerrieri, A. Quantification of l-lysine in cheese by a novel amperometric biosensor. Food Chem. 2015 , 169 , 13–19. [ Google Scholar ] [ CrossRef ]
- Strambini, L.M.; Longo, A.; Scarano, S.; Prescimone, T.; Palchetti, I.; Minunni, M.; Giannessi, D.; Barillaro, G. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens. Bioelectron. 2015 , 66 , 162–168. [ Google Scholar ] [ CrossRef ]
- Habtamu, H.B.; Ugo, P. Miniaturized Enzymatic Biosensor via Biofunctionalization of the Insulator of Nanoelectrode Ensembles. Electroanalysis 2015 , 27 , 2187–2193. [ Google Scholar ] [ CrossRef ]
- Malvano, F.; Albanese, D.; Sannini, A.; Crescitelli, A.; Pilloton, R.; Di Matteo, M. Ethanol content in must grape by alcohol dehydrogenase biosensors based on doped Polyaniline modified screen printed electrodes. Chem. Eng. Trans. 2015 , 43 , 37–42. [ Google Scholar ]
- Tomassetti, M.; Serone, M.; Angeloni, R.; Campanella, L.; Mazzone, E. Amperometric enzyme sensor to check the total antioxidant capacity of several mixed berries. Comparison with two other spectrophotometric and fluorimetric methods. Sensors 2015 , 15 , 3435–3452. [ Google Scholar ] [ CrossRef ]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015 , 67 , 214–223. [ Google Scholar ] [ CrossRef ]
- Tortolini, C.; Bollella, P.; Antiochia, R.; Favero, G.; Mazzei, F. Inhibition-based biosensor for atrazine detection. Sens. Actuators B Chem. 2016 , 224 , 552–558. [ Google Scholar ] [ CrossRef ]
- Grattieri, M.; Babanova, S.; Santoro, C.; Guerrini, E.; Trasatti, S.P.; Cristiani, P.; Bestetti, M.; Atanassov, P. Enzymatic Oxygen Microsensor Based on Bilirubin Oxidase Applied to Microbial Fuel Cells Analysis. Electroanalysis 2015 , 27 , 327–335. [ Google Scholar ] [ CrossRef ]
- Silletti, S.; Rodio, G.; Pezzotti, G.; Turemis, M.; Dragone, R.; Frazzoli, C.; Giardi, M.T. An optical biosensor based on a multiarray of enzymes for monitoring a large set of chemical classes in milk. Sens. Actuators B Chem. 2015 , 215 , 607–617. [ Google Scholar ] [ CrossRef ]
- Chiavaioli, F.; Biswas, P.; Trono, C.; Jana, S.; Bandyopadhyay, S.; Basumallick, N.; Giannetti, A.; Tombelli, S.; Bera, S.; Mallick, A.; et al. Sol-Gel-Based Titania-Silica Thin Film Overlay for Long Period Fiber Grating-Based Biosensors. Anal. Chem. 2015 , 87 , 12024–12031. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tardivo, M.; Toffoli, V.; Fracasso, G.; Borin, D.; Dal Zilio, S.; Colusso, A.; Carrato, S.; Scoles, G.; Meneghetti, M.; Colombatti, M.; et al. Parallel optical read-out of micromechanical pillars applied to prostate specific membrane antigen detection. Biosens. Bioelectron. 2015 , 72 , 393–399. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Casalini, S.; Dumitru, A.C.; Leonardi, F.; Bortolotti, C.A.; Herruzo, E.T.; Campana, A.; De Oliveira, R.F.; Cramer, T.; Garcia, R.; Biscarini, F. Multiscale sensing of antibody-antigen interactions by organic transistors and single-molecule force spectroscopy. ACS Nano 2015 , 9 , 5051–5062. [ Google Scholar ] [ CrossRef ]
- Zhao, L.; Han, H.; Ma, Z. Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: Addressing its conductivity and reproducibility challenges. Biosens. Bioelectron. 2018 , 101 , 304–310. [ Google Scholar ] [ CrossRef ]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015 , 52 , 9–18. [ Google Scholar ] [ CrossRef ]
- Scarano, S.; Dausse, E.; Crispo, F.; Toulmé, J.-J.; Minunni, M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta 2015 , 897 , 1–9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tintoré, M.; Mazzini, S.; Polito, L.; Marelli, M.; Latorre, A.; Somoza, Á.; Aviñó, A.; Fàbrega, C.; Eritja, R. Gold-coated superparamagnetic nanoparticles for single methyl discrimination in DNA aptamers. Int. J. Mol. Sci. 2015 , 16 , 27625–27639. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ravalli, A.; da Rocha, C.G.; Yamanaka, H.; Marrazza, G. A label-free electrochemical affisensor for cancer marker detection: The case of HER2. Bioelectrochemistry 2015 , 106 , 268–275. [ Google Scholar ] [ CrossRef ]
- Mulla, Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-modulated transistors detects odorant binding protein chiral interactions. Nat Comm. 2015 , 6 , 6010. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Palla-Papavlu, A.; Fernández-Pradas, J.M.; Serra, P.; Staiano, M.; Varriale, A.; D’Auria, S. A surface acoustic wave bio-electronic nose for detection of volatile odorant molecules. Biosens. Bioelectron. 2015 , 67 , 516–523. [ Google Scholar ] [ CrossRef ]
- Cennamo, N.; Giovanni, S.D.; Varriale, A.; Staiano, M.; Di Pietrantonio, F.; Notargiacomo, A.; Zeni, L.; D’Auria, S. Easy to use plastic optical fiber-based biosensor for detection of butanal. PLoS ONE 2015 , 10 , e0116770. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Compagnone, D.; Faieta, M.; Pizzoni, D.; di Natale, C.; Paolesse, R.; van Caelenberg, T.; Beheydtd, B.; Pittia, P. Quartz crystal microbalance gas sensor arrays for the quality control of chocolate. Sens. Actuators B Chem. 2015 , 207 , 1114–1120. [ Google Scholar ] [ CrossRef ]
- Zuccaro, L.; Tesauro, C.; Kurkina, T.; Fiorani, P.; Yu, H.K.; Knudsen, B.R.; Kern, K.; Desideri, A.; Balasubramanian, K. Real-Time Label-Free Direct Electronic Monitoring of Topoisomerase Enzyme Binding Kinetics on Graphene. ACS Nano 2015 , 9 , 11166–11176. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mariani, S.; Scarano, S.; Spadavecchia, J.; Minunni, M. A reusable optical biosensor for the ultrasensitive and selective detection of unamplified human genomic DNA with gold nanostars. Biosens. Bioelectron. 2015 , 74 , 981–988. [ Google Scholar ] [ CrossRef ]
- Lacina, K.; Sopoušek, J.; Čunderlová, V.; Hlaváček, A.; Václavek, T.; Lacinová, V. Biosensing based on electrochemical impedance spectroscopy: Influence of the often-ignored molecular charge. Electrochem. Commun. 2018 , 93 , 183–186. [ Google Scholar ] [ CrossRef ]
- Hamidi-Asl, E.; Raoof, J.B.; Hejazi, M.S.; Sharifi, S.; Golabi, S.M.; Palchetti, I.; Mascini, M. Genosensor for Point Mutation Detection of P53 Gene PCR Product Using Magnetic Particles. Electroanalysis 2015 , 27 , 1378–1386. [ Google Scholar ] [ CrossRef ]
- Wu, L.; Lu, X.; Fu, X.; Wu, L.; Liu, H. Gold nanoparticles dotted reduction Graphene oxide Nanocomposite based electrochemical Aptasensor for selective, rapid, sensitive and congener-specific PCB77 detection. Sci. Rep. 2017 , 7 , 1–7. [ Google Scholar ] [ CrossRef ]
- Khosla, A.; Shah, S.; Shiblee, M.N.I.; Mir, S.H.; Nagahara, L.A.; Thundat, T.; Shekar, P.K.; Kawakami, M.; Furukawa, H. Carbon fiber doped thermosetting elastomer for flexible sensors: Physical properties and microfabrication. Sci. Rep. 2018 , 8 , 1–8. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ahmad, R.; Khan, M.; Mishra, P.; Jahan, N.; Ahsan, M.A.; Ahmad, I.; Khan, M.R.; Watanabe, Y.; Syed, M.A.; Furukawa, H.; et al. Engineered hierarchical CuO nanoleaves based electrochemical nonenzymatic biosensor for glucose detection. J. Electrochem. Soc. 2021 , 168 , 017501. [ Google Scholar ] [ CrossRef ]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962 , 102 , 29–45. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, J.; Arya, S.; Verma, S.; Singh, A.; Sharma, A.; Singh, B.; Sharma, R. Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and Uric acid senor. Mater. Chem. Phys. 2019 , 238 , 121969. [ Google Scholar ] [ CrossRef ]
- Khan, M.; Nagal, V.; Nakate, U.T.; Khan, M.R.; Khosla, A.; Ahmad, R. Engineered CuO Nanofibers with Boosted Non-Enzymatic Glucose Sensing Performance. J. Electrochem. Soc. 2021 , 168 , 067507. [ Google Scholar ] [ CrossRef ]
- Tucci, M.; Grattieri, M.; Schievano, A.; Cristiani, P.; Minteer, S.D. Microbial amperometric biosensor for online herbicide detection: Photocurrent inhibition of Anabaena variabilis. Electrochim. Acta 2019 , 302 , 102–108. [ Google Scholar ] [ CrossRef ]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014 , 181 , 689–705. [ Google Scholar ] [ CrossRef ]
- Castillo-Ortega, M.M.; Rodriguez, D.E.; Encinas, J.C.; Plascencia, M.; Mendez-Velarde, F.A.; Olayo, R. Conductometric uric acid and urea biosensor prepared from electroconductive polyaniline–poly (n-butyl methacrylate) composites. Sens. Actuators B: Chem. 2002 , 85 , 19–25. [ Google Scholar ] [ CrossRef ]
- Sekhar, P.K.; Moore, Z.; Aravamudhan, S.; Khosla, A. A new low-temperature electrochemical hydrocarbon and NOx sensor. Sensors 2017 , 17 , 2759. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Li, A.; Khosla, A.; Drewbrook, C.; Gray, B.L. Fabrication and testing of thermally responsive hydrogel-based actuators using polymer heater elements for flexible microvalves. In Microfluidics, BioMEMS, and Medical Microsystems IX ; International Society for Optics and Photonics: San Francisco, CA, USA, 2011; Volume 7929, p. 79290G-1. [ Google Scholar ]
- Ahmad, R.; Tripathy, N.; Kim, S.H.; Umar, A.; Al-Hajry, A.; Hahn, Y.B. High performance cholesterol sensor based on ZnO nanotubes grown on Si/Ag electrodes. Electrochem. Commun. 2014 , 38 , 4–7. [ Google Scholar ] [ CrossRef ]
- Ahmad, R.; Tripathy, N.; Hahn, Y.B. High-performance cholesterol sensor based on the solution-gated field effect transistor fabricated with ZnO nanorods. Biosens. Bioelectron. 2013 , 45 , 281–286. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, C.; Tan, X.; Chen, S.; Yuan, R.; Hu, F.; Yuan, D.; Xiang, Y. Highly-sensitive cholesterol biosensor based on platinum-gold hybrid functionalized ZnO nanorods. Talanta 2012 , 94 , 263–270. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Batra, N.; Tomar, M.; Gupta, V. ZnO-CuO composite matrix based reagentless biosensor for detection of total cholesterol. Biosens. Bioelectron. 2015 , 67 , 263–271. [ Google Scholar ] [ CrossRef ]
- San Fang, C.; Oh, K.H.; Oh, A.; Lee, K.; Park, S.; Kim, S.; Yang, H. An ultrasensitive and incubation-free electrochemicalimmunosensor using a gold-nanocatalyst label mediating outer-spherereaction-philic and inner-sphere-reaction-philic species. Chem. Commun. 2016 , 52 , 5884–5887. [ Google Scholar ] [ CrossRef ]
- Cai, X.; Weng, S.; Guo, R.; Lin, L.; Chen, W.; Zheng, Z.; Lin, X. Ratiometric electrochemical immunoassay based on internalreference value for reproducible and sensitive detection of tumor marker. Biosens. Bioelectron. 2016 , 81 , 173–180. [ Google Scholar ] [ CrossRef ]
- Zhang, H.; Ma, L.; Li, P.; Zheng, J. A novel electrochemical immunosensor based onnonenzymatic Ag@au-Fe3O4 nanoelectrocatalyst for protein biomarkerdetection. Biosens. Bioelectron. 2016 , 85 , 343–350. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xu, L.; Zhu, Y.; Tang, L.; Yang, X.; Li, C. Biosensor based on self-assembling glucose oxidase and Dendrimer-encapsulated Pt nanoparticles on carbon nanotubes for glucosedetection. Electroanalysis 2007 , 19 , 717–722. [ Google Scholar ] [ CrossRef ]
- Dinh, D.; Hui, K.S.; Cho, Y.; Zhou, W.; Hong, X.; Chun, H.-H. Green synthesis of high conductivity silver nanoparticle-reduced graphene oxide composite films. Appl. Surf. Sci. 2014 , 298 , 62–67. [ Google Scholar ] [ CrossRef ]
- Gupta, S.; Kaushal, A.; Kumar, A.; Kumar, D. Ultrasensitive transglutaminase based nanosensor for early detection of celiac dis- ease in human. Int. J. Biol. Macromol. 2017 , 105 , 905–911. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Neves, M.M.; González-García, M.B.; Nouws, H.P.; Costa-García, A. Celiac disease detection using a transglutaminase electro-chemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens. Bioelectron. 2012 , 31 , 95–100. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Hianik, T.; Ostatná, V.; Zajacová, Z.; Stoikova, E.; Evtugyn, G.; Zajacova, Z.; Ostatna, V. Detection of aptamer—protein interactions using QCM and electrochemical indicator methods. Bioorg. Med. Chem. 2005 , 15 , 291–295. [ Google Scholar ] [ CrossRef ]
- Arya, S.; Singh, A.; Kour, R. Comparative study of CuO, CuO@ Ag and CuO@ Ag: La nanoparticles for their photosensing properties. Mater. Res. Express 2019 , 6 , 116313. [ Google Scholar ] [ CrossRef ]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010 , 28 , 232–254. [ Google Scholar ] [ CrossRef ]
- Hargunani, S.P.; Sonekar, R.P.; Singh, A.; Khosla, A.; Arya, S. Structural and spectral studies of Ce3+ doped Sr3Y (BO3) 3 nano phosphors prepared by combustion synthesis. Mater. Technol. 2020 , 1–12. [ Google Scholar ] [ CrossRef ]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010 , 39 , 1747–1763. [ Google Scholar ] [ CrossRef ]
- Marks, R.S.; Abdulhalim, I. Nanomaterials for Water Management: Signal Amplification for Biosensing from Nanostructures ; CRC Press: Boca Raton, FL, USA, 2015; Volume 4. [ Google Scholar ]
- Ferreira, A.; Fugivara, C.; Yamanaka, H.; Benedetti, A. Preparation and Characterization of Imunosensors for Disease Diagnosis. Biosens. Health Environ. Biosecurity 2011 , 540 . [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ly, S.Y.; Yoo, H.S.; Choa, S.H. Diagnosis of Helicobacter pylori bacterial infections using a voltammetric biosensor. J. Microbiol. Methods 2011 , 87 , 44–48. [ Google Scholar ] [ CrossRef ]
- Maalouf, R.; Chebib, H.; Saïkali, Y.; Vittori, O.; Sigaud, M.; Jaffrezic-Renault, N. Amperometric and impedimetric characterization of a glu-tamate biosensor based on Nafion and a methyl viologen modifi ed glassy carbon electrode. Biosens. Bioelectron. 2007 , 22 , 2682–2688. [ Google Scholar ] [ CrossRef ]
- Wang, J. Analytical Electrochemistry Hoboken ; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [ Google Scholar ]
- Elshafey, R.; Tavares, A.C.; Siaj, M.; Zouro, M. Electrochemical impedance immunosensor based on gold nanoparticles—protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue. Biosens. Bioelectron. 2013 , 50 , 143–149. [ Google Scholar ] [ CrossRef ]
- Helali, S.; Martelet, C.; Abdelghani, A.; Maaref, M.A.; Jaffrezic-Renault, N. A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads on gold surface for the detection of atrazine. Electrochim. Acta 2006 , 51 , 5182–5186. [ Google Scholar ] [ CrossRef ]
- Seven, B.; Bourourou, M.; Elouarzaki, K.; Constant, J.F.; Gondran, C.; Holzinger, M.; Cosnier, S.; Timur, S. Impedimetric biosensor for cancer cell detection. Electrochem. Commun. 2013 , 37 , 36–39. [ Google Scholar ] [ CrossRef ]
- Chowdhury, A.D.; De, A.; Chaudhuri, C.R.; Bandyopadhyay, K.; Sen, P. Label free polyaniline based impedimetric biosensor for detection of E. coli O157: H7 Bacteria. Sens. Actuators B Chem. 2012 , 171 , 916–923. [ Google Scholar ] [ CrossRef ]
- Rushworth, J.V.; Ahmed, A.; Griffiths, H.H.; Pollock, N.M.; Hooper, N.M.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 2014 , 56 , 83–90. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, N.; Lin, Y.; Yu, P.; Su, L.; Mao, L. Label-free and sequence-specific DNA detection down to apicomolar level with carbon nanotubes as support for probe DNA. Anal. Chim. Acta 2009 , 650 , 44–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shirsat, M.; Too, C.O.; Wallace, G. Amperometric glucose biosensor on layer bylayer assembled carbon nanotube and Polypyrrole multilayer film. Electroanalysis 2007 , 20 , 150–156. [ Google Scholar ] [ CrossRef ]
- Koncki, R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta 2007 , 599 , 7–15. [ Google Scholar ] [ CrossRef ]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdes-Ramirez, G.; Andrade, F.J.; Schoening, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014 , 54 , 603–609. [ Google Scholar ] [ CrossRef ]
- Papp, S.; Bojtar, M.; Gyurcsanyi, R.E.; Lindfors, T. Potential reproducibility of potassium-selective electrodes having perfluorinatedalkanoate side chain functionalized poly(3,4-ethylenedioxytiophene) as a hydrophobic solid contact. Anal. Chem. 2019 , 91 , 9111–9118. [ Google Scholar ] [ CrossRef ]
- Parrilla, M.; Cuartero, M.; Sanchez, S.P.; Rajabi, M.; Roxhed, N.; Niklaus, F.; Crespo, G.A. Wearable all-solid-state potentiometric microneedle patch for intradermal potassium detection. Anal. Chem. 2019 , 91 , 1578–1586. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens. Actuators B Chem. 2018 , 273 , 966–972. [ Google Scholar ] [ CrossRef ]
- Cánovas, R.; Blondeau, P.; Andrade, F.J. Modulating the mixed potential for developing biosensors: Direct potentiometric determination of glucose in whole, undiluted blood. Biosens. Bioelectron. 2020 , 163 , 112302. [ Google Scholar ] [ CrossRef ]
- Jakhar, S.; Pundir, C.S. Preparation, characterization and application of urease nanoparticles for construction of an improved potentiometric urea biosensor. Biosens. Bioelectron. 2018 , 100 , 242–250. [ Google Scholar ] [ CrossRef ]
- Scognamiglio, V.; Arduini, F.; Palleschi, G.; Rea, G. Bio sensing technology for sustainable food safety. Trends Anal. Chem. 2014 , 62 , 1–10. [ Google Scholar ] [ CrossRef ]
- Ghasemi-Varnamkhasti, M.; Rodriguez-Mendez, M.L.; Mohtasebi, S.S.; Apetrei, C.; Lozano, J.; Ahmadi, H.; Razavi, S.H.; de Saja, J.A. Monitoring the aging of beers using a bioelectronic tongue. Food Control. 2011 , 25 , 216–224. [ Google Scholar ] [ CrossRef ]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as innovative tools for the detection of food borne pathogens. Biosens. Bioelectron. 2011 , 28 , 1–12. [ Google Scholar ] [ CrossRef ]
- Ercole, C.; Del Gallo, M.; Mosiello, L.; Baccella, S.; Lepidi, A. Escherichia coli detection in vegetable food by a potentiometric biosensor. Sens. Actuators B Chem. 2003 , 91 , 163–168. [ Google Scholar ] [ CrossRef ]
- Torun, O.; Boyaci, I.; Temur, E.; Tamer, U. Comparison of sensing strategies in SPR biosensor for rapid and sensitive enumeration of bacteria. Biosens. Bioelectron. 2012 , 37 , 53–60. [ Google Scholar ] [ CrossRef ]
- Mishra, R.; Dominguez, R.; Bhand, S.; Munoz, R.; Marty, J. A novel Automated flow-based biosensor for the determination of organophosphate pesticides in milk. Biosens. Bioelectron. 2012 , 32 , 56–61. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yan, C.; Dong, F.; Chun-yuan, B.; Si-rong, Z.; Jian-guo, S. Recentprogress of commercially available biosensors in china and their applications in fermentation processes. J. Northeast. Agric Univ. 2014 , 21 , 73–85. [ Google Scholar ]
- Monosik, R.; Stredansky, M.; Tkac, J.; Sturdik, E. Application of enzyme biosensors in analysis of food and beverages enzyme and microbial technology. Food Anal. Methods 2012 , 5 , 40–53. [ Google Scholar ] [ CrossRef ]
- Arduini, F.; Ricci, F.; Tuta, C.S.; Moscone, D.; Amine, A.; Palleschi, G. Detection of carbamic and organophosphorus pesticides in water samples using cholinesterase biosensor based on Prussian blue modified screen printed electrode. Anal. Chim. Acta 2006 , 58 , 155–162. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, A.; Ahmed, A.; Sharma, A.; Sharma, C.; Paul, S.; Khosla, A.; Gupta, V.; Arya, S. Promising photocatalytic degradation of methyl orange dye via sol-gel synthesized Ag–CdS@ Pr-TiO2 core/shell nanoparticles. Phys. B Condens. Matter 2021 , 616 , 413121. [ Google Scholar ] [ CrossRef ]
- Suprun, E.; Evtugyn, G.; Budnikov, H.; Ricci, F.; Moscone, D.; Palleschi, G. Acetylcholinesterase sensor based on screenprinted carbon electrode modified with Prussian blue. Anal. Bioanal. Chem. 2005 , 383 , 597–604. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Diesel, E.; Schreiber, M.; van der Meer, J.R. Development of bacteria-based bioassays for arsenic detection in natural waters. Anal. Bioanal. Chem. 2009 , 394 , 687–693. [ Google Scholar ] [ CrossRef ]
- Scognamiglio, V.; Pezzotti, G.; Pezzotti, I.; Cano, J.; Buonasera, K.; Giannini, D.; Giardi, M.T. Biosensors for effective environmental and agrifood protection and commercialization: From research to market. Mikrochim. Acta 2010 , 170 , 215–225. [ Google Scholar ] [ CrossRef ]
- Rea, G.; Polticelli, F.; Antonacci, A.; Scognamiglio, V.; Katiyar, P.; Kulkarni, S.A.; Johanningmeier, U.; Giardi, M.T. Structure-based design of novelChlamydomonas reinhardtiiD1-D2 photosynthetic proteins for herbicide monitoring. Protein Sci. 2009 , 18 , 2139–2151. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A novel biosensor based on hafnium oxide: Application for early stage detection of human interleukin-10. Sens. Actuators B Chem. 2012 , 175 , 201–207. [ Google Scholar ] [ CrossRef ]
- Chen, Y.W.; Liu, M.; Kaneko, T.; McIntyre, P.C. Atomic layer deposited hafnium oxide gate dielectrics for charge-based biosensors. Electrochem. Solid State Lett. 2010 , 13 , G29–G32. [ Google Scholar ] [ CrossRef ]
- Ooi, K.G.J.; Galatowicz, G.; Towler, H.M.A.; Lightman, S.L.; Calder, V.L. Multiplex cytokine detection versus ELISA for aqueous humor: IL-5, IL-10, and IFN profiles in uveitis. Investig. Ophthalmol. Vis. Sci. 2006 , 47 , 272–277. [ Google Scholar ] [ CrossRef ]
- Caruso, R.; Trunfio, S.; Milazzo, F.; Campolo, J.; de Maria, R.; Colombo, T.; Parodi, O. Early expression of proand anti-inflammatory cytokines in left ventricular assist device recipients with multiple organ failure syndrome. Am. Soc. Art. Int. Org. J. 2010 , 56 , 313–318. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Caruso, R.; Verde, A.; Cabiati, M.; Milazzo, F.; Boroni, C.; Del Ry, S.; Parodi, O. Association of preoperative interleukin-6 levels with interagency registry for mechanically assisted circulatory support profiles and intensive care unit stay in left ventricular assist device patients. J Heart Lung Transplant. 2012 , 31 , 625–633. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Watson, C.J.; Ledwidge, M.T.; Phelan, D.; Collier, P.; Byrne, J.C.; Dunn, M.J.; McDonald, K.M.; Baugh, J.A. Proteomic Analysis of Coronary Sinus Serum Reveals Leucine-Rich α2-Glycoprotein as a Novel Biomarker of Ventricular Dysfunction and Heart Failure. Circ. Hear. Fail. 2011 , 4 , 188–197. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Chung, D.; Khosla, A.; Gray, B.L.; Parameswaran, A.M.; Ramaseshan, R.; Kohli, K. Investigations of flexible Ag/AgCl nanocomposite polymer electrodes for suitability in tissue electrical impedance scanning (EIS). J. Electrochem. Soc. 2013 , 161 , B3071. [ Google Scholar ] [ CrossRef ]
- Singh, A.; Arya, S.; Khanuja, M.; Hafiz, A.K.; Datt, R.; Gupta, V.; Khosla, A. Eu doped NaYF 4@ Er: TiO 2 nanoparticles for tunable ultraviolet light based anti-counterfeiting applications. Microsyst. Technol. 2020 , 1–10. [ Google Scholar ] [ CrossRef ]
- Morris, M.C. Fluorescent biosensors of intracellular targets from genetically encoded reporters to modular polypeptide probes. Cell Biochem. Biophys. 2010 , 56 , 19–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-protein biosensors: New tools for drug discovery. Trends Biotechnol. 1998 , 16 , 135–140. [ Google Scholar ] [ CrossRef ]
- Wolff, M.; Wiedenmann, J.; Nienhaus, G.U.; Valler, M.; Heilker, R. Novel fluorescent proteins for high-content screening. Drug Discov. Today 2006 , 11 , 1054–1060. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, J.; Arya, S.; Singh, A.; Verma, S.; Sharma, A.; Singh, B.; Tomar, A. Template Based Electrochemical Synthesis of Copper (Cu) Nanowires as CH2Cl2 Sensor. Integr. Ferroelectr. 2020 , 204 , 63–72. [ Google Scholar ] [ CrossRef ]
- El-Deiry, W.S.; Sigman, C.C.; Kelloff, G.J. Imaging and oncologic drug development. J Clin. Oncol. 2006 , 24 , 3261–3273. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au-SnO2 nanoparticles for electrochemical determination of vitamin B12. J. Mater. Res. Technol. 2020 , 9 , 14321–14337. [ Google Scholar ] [ CrossRef ]
- Morris, M.C. Fluorescent biosensors—probing protein kinase function in cancer and drug discovery. Biochim. Biophys. Acta 2013 , 1834 , 1387–1395. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Okumoto, S. Quantitative imaging using genetically encoded sensors for small molecules in plants. Plant J. 2012 , 70 , 108–117. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Choi, K.; Seo, W.; Cha, S.; Choi, J. Evaluation of two types of biosensors for immunoassay of botulinum toxin. J. Biochem. Mol. Biol. 1998 , 31 , 101–105. [ Google Scholar ]
- Thien, N.T.D.; Wang, H.; Sugiarto, S. Advances in nanomaterials and their applications in point of care (POC) devices for the diagnosis of infectious diseases. Biotechnol. Adv. 2016 , 34 , 1275–1288. [ Google Scholar ] [ CrossRef ]
- Davies, D.; Guo, X.; Musavi, L. Gold nanoparticle-modified carbon electrode biosensor for the detection of listeria monocytogenes. Ind. Biotechnol. 2013 , 9 , 13–36. [ Google Scholar ] [ CrossRef ]
- Yang, G.J.; Huang, J.L.; Menga, W.J. A reusable capacitive immunosensor for detection of Salmonella spp. based on grafted ethylene diamine and self-assembled gold nanoparticle monolayers. Anal. Chim. Acta 2009 , 647 , 159–166. [ Google Scholar ] [ CrossRef ]
- Gaffar, S.; Nurmalasari, R.; Yohan, Y.W. Voltammetric DNA biosensor using gold electrode modified by self assembled monolayer of Thiol for detection of mycobacterium tuberculosis. Procedia Technol. 2017 , 27 , 74–80. [ Google Scholar ] [ CrossRef ]
- Jampasa, S.; Wonsawat, W.; Rodthongkum, N.; Siangproh, W.; Yanatatsaneejit, P.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of human papillomavirus DNA type 16 using a pyrrolidinyl peptide nucleic acid probe immobilized on screen-printed carbon electrodes. Biosens. Bioelectron. 2014 , 54 , 428–434. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Topell, S.; Glockshuber, R. Circular permutation of the green fluorescent protein. Methods Mol. Biol. 2002 , 183 , 31–48. [ Google Scholar ]
- Tian, L.; Hires, S.A.; Looger, L.L. Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harb. Protoc. 2012 , 647–656. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Bermejo, C.; Ewald, J.C.; Lanquar, V.; Jones, A.M.; Frommer, W.B. In vivo biochemistry: Quantifying ion and metabolite levels in individual cells or cultures of yeast. Biochem. J. 2011 , 438 , 1–10. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Bermejo, C.; Haerizadeh, F.; Takanaga, H.; Chermak, D.; Frommer, W.B. Dynamic analysis of cytosolic glucose and ATP levels in yeast using optical sensors. Biochem. J. 2010 , 432 , 399–406. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Brett, C.L.; Kallay, L.; Hua, Z.; Green, R.; Chyou, A.; Zhang, Y.; Graham, T.R.; Donowitz, M.; Rao, R. Genome-wide analysis reveals the vacuolar pH-state of Saccharomyces cerevisiae. PLoS ONE 2011 , 6 , e17619. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Orij, R.; Urbanus, M.L.; Vizeacoumar, F.J.; Giaever, G.; Boone, C.; Nislow, C.; Brul, S.; Smits, G.J. Genome-wide analysis of intracellular pH reveals quantitative control of cell division rate by pHc in Saccharomyces cerevisiae. Genome Biol. 2012 , 13 , 1–15. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021 , 47 , 173–184. [ Google Scholar ] [ CrossRef ]
- Jamal, M.; Chakrabarty, S.; Shao, H.; McNulty, D.; Yousuf, M.A.; Furukawa, H.; Khosla, A.; Razeeb, K.M. A non enzymatic glutamate sensor based on nickel oxide nanoparticle. Microsyst. Technol. 2018 , 24 , 4217–4223. [ Google Scholar ] [ CrossRef ]
- Lussier, F.; Thibault, V.; Charron, B.; Wallace, G.Q.; Masson, J.-F. Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC Trends Anal. Chem. 2020 , 124 , 115796. [ Google Scholar ] [ CrossRef ]
- Dasgupta, A.; Nath, A. Classification of Machine Learning Algorithms. Int. J. Innov. Res. Adv. Eng. 2016 , 3 , 6–11. [ Google Scholar ]
- Mahesh, B. Machine Learning Algorithms-A Review. Int. J. Sci. Res. 2020 , 9 , 381–386. [ Google Scholar ]
- Yang, J.; Xu, J.; Zhang, X.; Wu, C.; Lin, T.; Ying, Y. Deep learning for vibrational spectral analysis: Recent progress and a practical guide. Anal. Chim. Acta 2019 , 1081 , 6–17. [ Google Scholar ] [ CrossRef ]
- Thrift, W.J.; Ragan, R. Quantification of Analyte Concentration in the Single Molecule Regime Using Convolutional Neural Networks. Anal. Chem. 2019 , 91 , 13337–13342. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. Rapid identification and quantification of illicit drugs on nanodendritic surface-enhanced Raman scattering substrates. Sens. Actuators B Chem. 2018 , 257 , 382–388. [ Google Scholar ] [ CrossRef ]
- Doty, K.C.; Lednev, I.K. Differentiation of human blood from animal blood using Raman spectroscopy: A survey of forensically relevant species. Forensic Sci. Int. 2018 , 282 , 204–210. [ Google Scholar ] [ CrossRef ]
- Shin, H.; Oh, S.; Hong, S.; Kang, M.; Kang, D.; Ji, Y.-g.; Choi, B.H.; Kang, K.-W.; Jeong, H.; Park, Y. Early-Stage Lung Cancer Diagnosis by Deep Learning-Based Spectroscopic Analysis of Circulating Exosomes. ACS Nano 2020 , 14 , 5435–5444. [ Google Scholar ] [ CrossRef ]
- Xu, Y.; Zomer, S.; Brereton, R.G. Support vector machines: A recent method for classification in chemometrics. Crit. Rev. Anal. Chem. 2006 , 36 , 177–188. [ Google Scholar ] [ CrossRef ]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A library for support vector machines, ACM Trans. Intell. Syst. Technol. 2011 , 2 , 1–27. [ Google Scholar ] [ CrossRef ]
- Vapnik, V. The Nature of Statistical Learning Theory ; Springer: Berlin/Heidelberg, Germany, 1995; ISBN 0-387-94559-8. [ Google Scholar ]
- Burges, C. A tutorial on support vector machines for pattern recognition. In Data Mining and Knowledge Discovery ; Kluwer Academic Publishers: Boston, MA, USA, 1998; Volume 2. [ Google Scholar ]
- Vapnik, V.; Golowich, S.; Smola, A. Support vector method for function approximation, regression estimation, and signal processing. In Advances in Neural Information Processing Systems 9 ; Mozer, M., Jordan, M., Petsche, T., Eds.; MIT Press: Cambridge, MA, USA, 1997; pp. 281–287. [ Google Scholar ]
- Wang, C.; Madiyar, F.; Yu, C.; Li, J. Detection of extremely low concentration waterborne pathogen using a multiplexing selfreferencing SERS microfluidic biosensor. J. Biol. Eng. 2017 , 11 , 9. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Majumder, S.; Ghosh, N.; Gupta, P. Support vector machine fornoptical diagnosis of cancer. J. Biomed. Opt. 2005 , 10 , 024034. [ Google Scholar ] [ CrossRef ]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput. Biol. Med. 2018 , 100 , 270–278. [ Google Scholar ] [ CrossRef ]
- Olano, X.; Sallaberry, F.; García De Jalón, A.; Gastón, M. The influence of sky conditions on the standardized calibration of pyranometers and on the measurement of global solar irradiation. Sol. Energy 2015 , 121 , 116–122. [ Google Scholar ] [ CrossRef ]
- Liu, J.; Fang, W.; Zhang, X.; Yang, C. An improved photovoltaic power forecasting model with the assistance of aerosol index data. IEEE Trans. Sustain. Energy 2015 , 6 , 434–442. [ Google Scholar ] [ CrossRef ]
- Asefpour Vakilian, K.; Massah, J. An artificial neural network approach to identify fungal diseases of cucumber (Cucumis sativus L.) plants using digital image processing. Arch. Phytopathol. Plant Protect. 2013 , 46 , 1580–1588. [ Google Scholar ] [ CrossRef ]
- Kallenberg, M.; Petersen, K.; Nielsen, M.; Ng, A.Y.; Diao, P.; Igel, C.; Vachon, C.M.; Holland, K.; Winkel, R.R.; Karssemeijer, N. Unsupervised deep learning applied to breast density segmentation and mammographic risk scoring. IEEE Trans. Med. Imaging 2016 , 35 , 1322–1331. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C.A. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans. Med. Imaging 2016 , 35 , 1240–1251. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Setio, A.A.A.; Ciompi, F.; Litjens, G.; Gerke, P.; Jacobs, C.; Van Riel, S.J.; Wille, M.M.W.; Naqibullah, M.; Sánchez, C.I.; van Ginneken, B. Pulmonary nodule detection in CT images: False positive reduction using multi-view convolutional networks. IEEE Trans. Med. Imaging 2016 , 35 , 1160–1169. [ Google Scholar ] [ CrossRef ]
- Tahir, A.M.; Chowdhury, M.E.H.; Khandakar, A.; Al-Hamouz, S.; Abdalla, M.; Awadallah, S.; Reaz, M.B.I.; Al-Emadi, N. A Systematic Approach to the Design and Characterization of a Smart Insole for Detecting Vertical Ground Reaction Force (vGRF) in Gait Analysis. Sensors 2020 , 20 , 957. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Pdf ImageNet classification with deep convolutional neural networks. Commun. ACM 2017 , 60 , 84–90. [ Google Scholar ] [ CrossRef ]
- Ho, T.K.K.; Gwak, J. Multiple Feature Integration for Classification of Thoracic Disease in Chest Radiography. Appl. Sci. 2019 , 9 , 4130. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liang, G.; Zheng, L. A transfer learning method with deep residual network for pediatric pneumonia diagnosis. Comput. Methods Programs Biomed. 2020 , 187 , 104964. [ Google Scholar ] [ CrossRef ]
- Virkki, R.; Juvén, T.; Rikalainen, H.; Svedstrom, E.; Mertsola, J.; Ruuskanen, O. Differentiation of bacterial and viral pneumonia in children. Thorax 2002 , 57 , 438–441. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Goyal, M.; Goyal, R.; Lall, B. Learning Activation Functions: A new paradigm of understanding Neural Networks. arXiv 2019 , arXiv:1906.09529. [ Google Scholar ]
- Bailer, C.; Habtegebrial, T.; Varanasi, K.; Stricker, D. Fast Feature Extraction with CNNs with Pooling Layers. arXiv 2018 , arXiv:1805.03096. [ Google Scholar ]
- Lipton, Z.C.; Berkowitz, J.; Elkan, C. A critical review of recurrent neural networks for sequence learning. arXiv 2015 , arXiv:1506.00019. [ Google Scholar ]
- Ordóñez, F.J.; Roggen, D. Deep convolutional and lstm recurrent neural networks for multimodal wearable activity recognition. Sensors 2016 , 16 , 115. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kiddon, C.; Zettlemoyer, L.; Choi, Y. Globally coherent text generation with neural checklist models. In Proceedings of the 2016 Conference on Empirical Methods in Natural Language Processing, Austin, TX, USA, 1–5 November 2016; pp. 329–339. [ Google Scholar ]
- Takeuchi, D.; Yatabe, K.; Koizumi, Y.; Oikawa, Y.; Harada, N. Real-time speech enhancement using equilibriated RNN. In Proceedings of the ICASSP 2020−2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain, 4−8 May 2020. [ Google Scholar ]
- Doetsch, P.; Kozielski, M.; Ney, H. Fast and robust training of recurrent NEURAL networks for offline handwriting recognition. In Proceedings of the 2014 14th International Conference on Frontiers in Handwriting Recognition, Hersonissos, Greece, 1−4 September 2014; pp. 279–284. [ Google Scholar ]
- Williams, J.D.; Zweig, G. End-to-end lstm-based dialog control optimized with supervised and reinforcement learning. arXiv 2016 , arXiv:1606.01269. [ Google Scholar ]
- Corbett, P.; Boyle, J. Improving the learning of chemicalprotein interactions from literature using transfer learning and specialized word embeddings. Database 2018 , 2018 , 1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, X.; Wu, Y.; Song, G.; Li, Z.; Fan, Y.; Zhang, Y. Brain Tumor Segmentation Using a Fully Convolutional Neural Network with Conditional Random Fields, International Workshop on Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries ; Springer: Berlin/Heidelberg, Germany, 2016; pp. 75–87. [ Google Scholar ]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997 , 9 , 1735–1780. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, Q.; Fang, L.; Yu, G.; Wang, D.; Xiao, C.-L.; Wang, K. Detection of DNA base modifications by deep recurrent neural network on Oxford Nanopore sequencing data. Nat. Commun. 2019 , 10 , 1–11. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Rang, F.J.; Kloosterman, W.P.; de Ridder, J. From squiggle to basepair: Computational approaches for improving nanopore sequencing read accuracy. Genome Biol. 2018 , 19 , 90. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Available online: https://www.dimensions.ai/ (accessed on 12 August 2021).
- Viswanathan, S.; Rani, C.; Anand, A.V.; Ho, J.A.A. Disposable electrochemical immunosensor forcarcinoembryonic antigen using ferrocene liposomes and MWCNT screenprinted electrode. Biosens. Bioelectron. 2009 , 24 , 1984–1989. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Method | Target | Biological Element | Target Matrix | Transducer Element | Ref. |
|---|---|---|---|---|---|
| Amperometric | Cholesterol | Cholesterol oxidase | Human serum | Prussian Blue modified SPE | [ ] |
| Amperometric | Lactate | Lactate oxidase | Wine | Prussian Blue modified SPE | [ ] |
| Amperometric | Polyamines | Polyamine oxidase, spermine oxidase | Food | Prussian Blue modified SPE | [ ] |
| Amperometric | Lysine | Lysine oxidase | Cheese | Pt electrode | [ ] |
| Amperometric | Glucose | Glucose oxidase | Transdermal fluid | Transdermal microneedles | [ ] |
| Amperometric | Glucose | Glucose oxidase | - | Gold nanoelectrode | [ ] |
| Amperometric | Ethanol | Alcohol dehydrogenase | wine | Polyaniline doped modified SPE | [ ] |
| Amperometric | Antioxidant capacity | Superoxide disumlase | Fruit juice and berries | Pt electrode | [ ] |
| Amperometric differential | Antioxidant capacity+ ascorbate | Ascorbate oxidase | Fruit juice | Fullerene modified graphite | [ ] |
| Amperometric inhibition | Atrazine | Tyrosinase | Drinking water | Carbon modified SPE | [ ] |
| Amperometric | Oxygen profile | Biliribine oxidase | Microbial fuel cell | Pt electrode | [ ] |
| Label-free evanescent wave | IgG | Antibody | Human serum | Titania–silica-coated long period gratings optical fibers | [ ] |
| Label-free CCD + software for imaging | Prostate specific antigen | Antibody | Human serum | Dense arrays of micropillars | [ ] |
| Label-free field effect transistor | Interleukin 4 | Antibody | Human serum | Organic transistor | [ ] |
| Voltametric/ impedimetric | Aflatoxin B1 | Aptamer | Peanuts and peanuts corn snacks | Dendrimer- modified gold electrode | [ ] |
| Label-free, piezoelectric using 2 different aptamers | Metalloproteinase 9 | Aptamers | Human serum | Quartz crystal microbalance | [ ] |
| Colorimetric, aggregation using 2 aptamers | DNA methylation | Aptamers for α-thrombin | DNA | Au coated magnetic nanoparticles | [ ] |
| Impedimetric | Human epidermal growth factor receptor 2 | Antibody | Human serum | Au–nano-particles on SPE | [ ] |
| : MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021 , 11 , 336. https://doi.org/10.3390/bios11090336
Singh A, Sharma A, Ahmed A, Sundramoorthy AK, Furukawa H, Arya S, Khosla A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors . 2021; 11(9):336. https://doi.org/10.3390/bios11090336
Singh, Anoop, Asha Sharma, Aamir Ahmed, Ashok K. Sundramoorthy, Hidemitsu Furukawa, Sandeep Arya, and Ajit Khosla. 2021. "Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope" Biosensors 11, no. 9: 336. https://doi.org/10.3390/bios11090336
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

COMMENTS
A biosensor is an integrated receptor-transducer device, which can convert a biological response into an electrical signal. The design and development of biosensors have taken a center stage for researchers or scientists in the recent decade owing to the wide range of biosensor applications, such as health care and disease diagnosis, environmental monitoring, water and food quality monitoring ...
Biosensors consist of three parts: a component that recognizes the analyte and produces a signal, a signal transducer, and a reader device. Latest Research and Reviews
This review provides a comprehensive study of biosensors and covers almost all the relevant topics, including components, working, and parameters. ... (DPI) in one of the research papers [127]. The DPI-based Cd 2+ aptasensor device can quantify the concentration of Cd 2+ with high sensitivity and good selectivity. During interaction with the ...
The major application of microbial biosensors is in the environmental field [4 and 15 ]. Environmental applications of biosensors include the detection of harmful bacteria or. pesticides in air ...
Now, through molecular-level design, a leakage-free and creep-free polyelectrolyte elastomer is synthesized, and an iontronic sensor using the polyelectrolyte elastomer shows very low signal drift ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Biosensors is an international ...
The capability and diversity of biosensors for human healthcare have grown tremendously, with novel approaches continually being developed. Increased sensitivities of biosensors for in vitro diagnosis have been achieved using nanomaterials and nanotechnologies, while paper-based biosensor devices are a cost-effective alternative, all while maintaining high sensitivities and adapting ingenious ...
Abstract. Nanophotonic devices, which control light in subwavelength volumes and enhance light-matter interactions, have opened up exciting prospects for biosensing. Numerous nanophotonic ...
Fabrication of biosensors, its materials, transducing devices, and immobilization methods requires multidisciplinary research in chemistry, biology, and engineering. The materials used in biosensors are categorized into three groups based on their mechanisms: biocatalytic group comprising enzymes, bioaffinity group including antibodies and ...
A biosensor is an integrated receptor-transducer device, which can convert a biological response into an electrical signal. The design and development of biosensors have taken a center stage for researchers or scientists in the recent decade owing to the wide range of biosensor applications, such as health care and disease diagnosis, environmental monitoring, water and food quality monitoring ...
Journal of Materials Research and Technology. Volume 12, May-June 2021, Pages 1649-1672. Review Article. ... In biosensors, the part of the system used to attach to the analyte and specifically detect it is a biological element (such as a DNA strand, antibody, enzyme, whole cell). The "Nano Biosensors" series reviews various types of ...
5. Nanomaterials for Biosensors. The evolution of research on biosensors has gained attention among researchers with its potential utilization in industrial applications and scientific fields including bioinformatics, biotechnology, electronics, materials science, healthcare, medical science, and so on . A biosensor is a device that can sense a ...
Read the latest Research articles in Biosensors from Scientific Reports. ... Highly sensitive label-free biosensor: graphene/CaF 2 multilayer for gas, cancer, ... Calls for Papers
Biosensors are the devices that capture the biological signal and convert it into a detectable electrical signal. It involves the combination of biological entities like DNA, RNA, and proteins ...
Biosensors are devices that combine a biological material with a suitable platform for detection of pathogenic organisms, carcinogenic, mutagenic, and/or toxic chemicals or for reporting a biological effect. In recent years, an enormous number of different types of biosensors have been constructed and developed for several medical applications.
This paper discusses biosensors and their significant benefits in the medical field. Distinctive capabilities of biosensors in healthcare services and for cardiovascular disease are provided and shown diagrammatically. ... Biosensors research is attracting enormous interest from its application in clinical treatment, pharmacy, biomedical and ...
Abstract. This paper reviews sensors with nano- and microscale dimensions used for diverse biological applications. A biosensor converts biological responses into electrical signals. In recent years, there have been significant advancements in the design and development of biosensors that generated a large spectrum of biosensor applications including healthcare, disease diagnosis, drug ...
A Pt electrode printed on paper was used along with a polyelectrolyte called aquivion. The polyelectrolyte helps in entrapping the glucose enzyme and reducing interference and potential instability. ... The development and research in electrochemical biosensors are becoming popular in biology, electronics, material science, and engineering. ...
Wearable biosensors are garnering substantial interest due to their potential to provide continuous, real-time physiological information via dynamic, noninvasive measurements of biochemical ...
This need explains the recent trends in the development of biosensing devices for pollutant detection. The present review aims to summarize the newest trends regarding the use of biosensors to detect environmental contaminants. Enzyme, whole cell, antibody, aptamer, and DNA-based biosensors and biomimetic sensors are discussed.
Batch fabrication and integration of disposable, flexible and multi-electrode electrochemical biosensors with different substrates, including plastics, flexible films, textiles and paper, can be ...
Highly Sensitive Biosensors Based on All-PEDOT:PSS Organic Electrochemical Transistors with Laser-Induced Micropatterning ... Article keywords are supplied by the authors and highlight key terms and topics of the paper. organic electrochemical transistor ; electrochemical sensing ... Such files may be downloaded by article for research use (if ...
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... The development and research in electrochemical biosensors are becoming popular in biology, electronics, material science, and engineering ...
Sensitivity and Linearity: The biosensors are rated high only if they possess high sensitivity. In today's world especially in air, water and soil pollutant detection, the requirement is a ppm level whereas in medical field it goes from nanograms per milliliter to femtograms per milliliter [13].Further linearity of the device highlights the accuracy of the given response for a given set of ...